Abstract
Placental mesenchymal dysplasia (PMD) is a relatively recently recognized, rare placental vascular anomaly characterized by placentomegaly and grapelike vesicles that may coexist with a normal fetus. Ultrasonography performed at 16 weeks of gestation revealed multiple cysts in the placenta along with a morphologically normal fetus. At 24 weeks of gestational age, severe preeclampsia symptoms were seen. And sonographically intrauterine growth restriction (IUGR) and oligohydroamnios were noted. Based on the diagnosis of severe preeclampsia, termination of pregnancy was done. After termination, histopathologic examination of the placenta showed numerous grape-like cystic vesicles throughout the entire placenta and absence of trophoblastic proliferation and pseudoinclusions. We have described a pregnancy complicated with PMD associated with mid-trimester severe preeclampsia, IUGR and oligohydroamnios.
Placental mesenchymal dysplasia (PMD) is an uncommon disorder in which the placenta is enlarged with abnormal, large and cystic villi with dilated, thick-walled vessels. These placental changes can mimic a partial hydatidiform mole. A thickened placenta with hypoechoic spaces are classical sonographic findings of both PMD and molar pregnancies [1].
Prenatal recognition of PMD during early as well as late gestation could prevent unnecessary termination of pregnancy. Placental mesenchymal dysplasia in early pregnancy is more likely to be mistaken for a partial mole especially by ultrasonographic examination.
In partial hydatidiform mole, the placenta is characterized by 2D sonography as heterogeneous, partially solid with cystic areas, representing hydropic villous degeneration of the molar tissue, resembling the sonographic appearance of PMD. Color Doppler studies of a partial mole placenta can demonstrate the presence of high-velocity, low impedance flow with low resistance indices in the molar mass. Thus, although PMD may be associated with different patterns of blood flow with advancing gestation, the absent or low venous signals inside the placental lesion may be of value in differentiating PMD from a molar pregnancy that are characterized by high velocity blood flow.
Histologically, these placentas could be distinguished from partial moles because of the absence of trophoblastic proliferation.
Ultrasonographic findings suggestive of a molar pregnancy because of hypoechoic spaces in the placenta in the presence of an apparently normal fetus, a fetus with growth restriction, or a fetus with features of overgrowth should raise the possibility of PMD. Unlike molar pregnancies, characterized by absent or malformed fetuses, the pregnancy often extends into the third trimester in PMD. So once we consider PMD, management of the pregnancy is to observe closely concerning any complications for successful pregnancy outcome.
There is no specific clinical symptomatology associated with PMD. Most cases of PMD in early pregnancy are diagnosed by prenatal ultrasonography done either for routine prenatal checkup or because of an abnormal 2nd-trimester serum screening test.
The most common abnormal laboratory test includes increased level of maternal serum alpha fetoprotein, which is thought to be of fetal origin [1]. The level of human chorionic gonadotropin is normal to slightly increased but returns to normal levels soon after delivery [2].
Later in the pregnancy, the patient usually presents with intrauterine growth restriction (IUGR) or fetal demise. Patients may also present with polyhydramnios if the fetus has swallowing difficulty as part of Beckwith-Wiedemann syndrome (BWS).
Severe IUGR in PMD may be related to diversion of fetal blood within the vascular malformation or stem villi blood vessel thrombosis resulting in hypoperfusion and hypoxia that ultimately leads to IUGR [3].
Although many obstetrical complications such as fetal hydrops, gestational diabetes, and preeclampsia may be associated with a large placenta, placentomegaly in PMD is thought not to be the cause of obstetrical complications. No direct associations of placental weight and fetal or maternal complications have been identified. Rather, the complications, such as IUGR and preeclampsia are thought to be related to the degree of vascularity and excessive vascular shunting into the chorangiomatous areas [4].
Although the precise mechanism of preeclampsia was remained elusive, accumulating evidence has indicated that placental dysfunction, endothelial dysfunction, oxidative stress and the inflammatory response may participate in the development of preeclampsia.
We present a pregnancy complicated with PMD, along with a review of the literature.
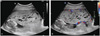
A 39-year-old woman, para 0, was referred to our hospital at 16+3/7 weeks of gestation because of a placental abnormality seen on routine sonographic examination. Ultrasonography performed at our institution revealed an intrauterine singleton pregnancy with a morphologically normal fetus. Uterine and umbilical artery pulsatility index and resistance index measurements were within the normal range. We also found a placental abnormality with multiple, variable sized cysts occupying whole placenta (Fig. 1A). Color Doppler examination failed to detect any blood flow signal within the mass (Fig. 1B). To differentiate with partial mole, the quad test and amniocentesis were recommended.
The quad test performed at 16+3/7 weeks of gestation showed significantly raised alpha fetoprotein (AFP) to 501.51 ng/mL (13.184 MoM), inhibin A of 2602.0 pg/mL (13.924 MoM), with slightly raised human chorionic gonadotropin of 246.70 IU/mL (7.469 MoM), unconjugated estriol of 0.779 ng/mL (0.731 MoM) within normal limit.
Amniocentesis was failed due to the placental position, so chorionic villous sampling (CVS) was conducted. CVS at 18+3/7 weeks of gestation showed a normal female diploid (46XX).
We considered PMD because of sonographic findings of a placental lesion, a fetus with a normal karyotype, and abnormal serum test including significantly raised AFP level.
Serial sonographic examinations performed every 2-3 weeks showed that the placental lesion gradually increased in size, that the fetus became small for gestational age.
Sonographic examination at 20+2/7 weeks of gestation showed that the amniotic fluid volume was lower than the normal range (maximal vertical pocket: 1.8). The ultrasound-based estimated fetal weight was 211 g, below the 5th percentiles for that gestational age.
At 24+6/7 weeks of gestation, the pregnant woman complained of a headache. Her blood pressure was 160/100 mm Hg, and had a severe proteinuria, severe pitting edema in limbs. Her chest X-ray showed bilateral pleural effusion. Aspartate aminotransferase, alanine aminotransferase were 46, 56. And platelet count was 76,000. Sonographic examination presented placentomegaly with multiple small cysts. The ultrasound-based estimated fetal weight was 22+4/7 weeks of gestation size. The amniotic fluid index was 4.6. Based on the diagnosis of a severe form of preeclampsia, oligohydroamnios and IUGR, and that we could not rule out the possibility of the placental molar change with confined placental mosaicism due to early onset of preeclampsia, termination of pregnancy was decided.
Labor was induced and intravenous magnesium sulfate was given to the patient. And her blood pressure was controlled with intravenous hydralazine. A female dead fetus was delivered vaginally.
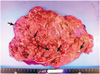
The delivered placenta was markedly large (36 × 22 × 4 cm, 980 g) for that gestational age with a 19 cm long umbilical cord attached. The vessels on the chorionic plate are tortuous and dilated. The placental parenchyma shows numerous grape-like cystic vesicles throughout the entire placenta (Fig. 2).
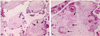
Microscopically, the thick-walled chorionic plate vessels showed fibromuscular hyperplasia, fresh or organizing thrombi, some degree of fibrinoid necrosis. Enlarged stem villi had central cisterns and loosed myxoid stroma. Villous vessels were partially or completely obliterated with thrombosis and fibrin deposit (Fig. 3). Neither abnormal trophoblastic proliferation nor trophoblastic pseudoinclusions were observed.
Placental mesenchymal dysplasia is a rare disease entity. Sonographically, PMD shows findings similar to those of partial hydatidiform mole, such as enlarged placentas with multicystic, anechoic regions, and widely distributed, large, edematous villi as seen under gross examination. PMD has distinct clinicopathologic features. Unlike molar pregnancies, characterized by absent or malformed fetuses, PMD usually features a normal fetus and the pregnancy often extends into the third trimester.
It is difficult to determine whether the complications faced by these fetuses are true complications of this disease process or merely coincidental findings.
According to the 82 PMD cases identified until 2005, among all cases without BWS, 50% had IUGR and 43% had IUFD or neonatal death. Females represented 82% of cases. PMD is associated with high IUGR and IUFD/neonatal death rates and disproportionally affects females [5].
Chromosomal abnormalities may be found in a few fetuses, but most are karyotypically and phenotypically normal females. Placental mesenchymal dysplasia may be associated with chromosomal abnormalities such as trisomy 13, Klinefelter syndrome, triploidy and Xp deletion [6,7]. Rare cases of fetal congenital adrenal hyperplasia, vascular hamartoma, and hepatic mesenchymal hamartoma have been described [8].
Women who are carrying a trisomy 13 fetus are prone to have an abnormal placenta as well as to develop preeclampsia in the second and third trimesters with the histopathologic findings of abnormal trophoblastic invasion into the uterine spiral arteries [2,9]. There were 4 cases of PMD with preeclampsia among 82 PMD cases identified until 2005. Three cases were chromosomally normal, 1 case did not analyze chromosome. Two cases were IUFD and they had BWS, and 1 case got therapeutic abortion and had a probability of having BWS [5].
In ongoing pregnancies, the absence of any relationship between the placental size and weight and a specific fetal or maternal complication suggests that the size of the placenta has little influence on the clinical outcome. Rather, the complications are thought to be related to the degree of vascularity and excessive vascular shunting into the chorangiomatous areas [4].
The central factor in the pathogenesis of preeclampsia appears to be placental ischemia which is supposed to be caused by an initial defective placentation. The impaired placental function leads to generalized endothelial cell dysfunction, which in its turn gives rise to hypertension, proteinuria, edema, thrombocytopenia and hypoperfusion, especially of liver and kidneys. The degree of vascularity and excessive vascular shunting into the chorangiomatous areas, stem villi blood vessel thrombosis seem to be associated with the occurrence of preeclampsia in PMD.
In this case, the vessels on the chorionic plate are tortuous and dilated, villous vessels were partially or completely obliterated with thrombosis. Also there were numerous cystic vesicles throughout the entire placenta. Malperfusion of the placenta resulting from this poor vascularity seem to be a cause of IUGR, oligohydroamnios and severe preeclampsia in second trimester.
We have described a pregnancy complicated with PMD and this was associated with mid-trimester severe preeclampsia, IUGR and oligohydroamnios.
Figures and Tables
Fig. 1
Transabdominal ultrasound examinations at (A), (B) 16 weeks and showing a thickened placenta with multiple, variable sized cysts, no blood flow signal within the mass.
References
1. Moscoso G, Jauniaux E, Hustin J. Placental vascular anomaly with diffuse mesenchymal stem villous hyperplasia. A new clinico-pathological entity? Pathol Res Pract. 1991. 187:324–328.
2. Feinberg RF, Kliman HJ, Cohen AW. Preeclampsia, trisomy 13, and the placental bed. Obstet Gynecol. 1991. 78:505–508.
3. Kuwabara Y, Shima Y, Araki T, Shin S. Mesenchymal stem villous hyperplasia of the placenta and fetal growth restriction. Obstet Gynecol. 2001. 98:940–943.
4. Jauniaux E, Nicolaides KH, Hustin J. Perinatal features associated with placental mesenchymal dysplasia. Placenta. 1997. 18:701–706.
5. Pham T, Steele J, Stayboldt C, Chan L, Benirschke K. Placental mesenchymal dysplasia is associated with high rates of intrauterine growth restriction and fetal demise: A report of 11 new cases and a review of the literature. Am J Clin Pathol. 2006. 126:67–78.
6. Arizawa M, Nakayama M. Suspected involvement of the X chromosome in placental mesenchymal dysplasia. Congenit Anom (Kyoto). 2002. 42:309–317.
7. Müngen E, Dundar O, Muhcu M, Haholu A, Tunca Y. Placental mesenchymal dysplasia associated with trisomy 13: sonographic findings. J Clin Ultrasound. 2008. 36:454–456.
8. Carta M, Maresi E, Giuffrè M, Catalano G, Piro E, Siracusa F, et al. Congenital hepatic mesenchymal hamartoma associated with mesenchymal stem villous hyperplasia of the placenta: case report. J Pediatr Surg. 2005. 40:e37–e39.
9. Chen CP. Placental abnormalities and preeclampsia in trisomy 13 pregnancies. Taiwan J Obstet Gynecol. 2009. 48:3–8.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download