Abstract
Objective
Human papillomavirus (HPV) is known as the causal agent of cervical cancer. The Papanicolaou smear is the most popular screening tool for cervical cancer. Some cytological low grade squamous intraepithelial lesions (LSILs) are in the developmental stage of high grade intraepithelial lesions (HSILs) and cervical cancer metaphases. Our purpose is to disclose the significance of high risk type (HRT) HPV in LSIL.
Methods
Documentation of 200 cases of cone biopsy at Hallym University Sacred Heart Hospital from February 2006 to February 2008 was reviewed retrospectively. HPV typing with DNA microarray results were found in 20 of the LSIL patients. Chi-square and student-t tests were used in the statistical analysis.
Results
In the cytological LSIL patients, HRT-HPV positive patients were 16/20 (80%) and one low risk type (LRT) HPV positive patient was 1/20 (5%). In the cytological LSIL patients, postoperative pathological diagnoses were cervical intraepithelial neoplasia 1 (CIN 1) 16/26 (61.5%), CIN2 2/26 (7.7%), CIN3 5/26 (19.2%), carcinoma in situ 1/26 (3.8%). Among the 16 HRT-HPV positive patients, there were six that were above HSIL 6/16 (37.5%) in pathologic diagnoses. In the HRT-HPV negative 10 cytologic LSIL patients, there were two that were above HSIL 2/10 (20%) in pathologic diagnoses. HRT-HPV prevalences were not significantly different between LSIL and HSIL.
Human papillomavirus (HPV) is known as the causal agent of cervical cancer. The Papanicolaou smear is the most popular screening tool for cervical cancer. Some low grade squamous intraepithelial lesions (LSILs) are the metaphase of the course going to high grade squamous intraepithelial lesion (HSIL) and probably will be presented as HSIL or cancer in the future.
Although screening methods have been improved in cytology, both conventional and liquid paps show relatively little difference in accuracy [1]. The sensitivity of cytology is 70-80% [2,3]. It is known that LSIL persists in 32% and progresses to carcinoma in situ (CIS) in 11% [4]. The HPV test is helpful in making decisions in cervical cytology. It is known that LSILs are related to low risk HPV types 6, 11, 40, 42, 43, 44, 54, 61, 70, 72, 81 and HSILs and cervical cancers are related to high risk HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73 and 82. But some cervical intraepithelial neoplasia 2 (CIN 2) and 3 lesions develop without a preexisting CIN 1 [5,6]. And HPVs are positive in CIN 1 about 85% [7,8]. Some CIN 1s and almost all CIN 2 and 3 lesions are associated with monoclonal "high-risk" HPV types [5,9].
For clinical convenience, cytological LSIL should be proven as pathological LSIL and cytological HSIL should be proven as pathological HSIL. But we could not agree to utilize such a rule in the clinic. The results of LSILs and HSILs in cytology are not always pathologic LSILs and HSILs. In cytological HSILs, the next step is simple. Cone biopsies always follow the cytologic HSILs. In cytological LSIL, it is necessary to make a decision between the cone biopsy or the follow-up.
With the viewpoint that guidance is necessary in LSIL, HPV testing is one of the best choices for gynecologists before colposcopic consultation. A repeat pap follow up and immediate colposcopy are recommended as alternatives, if available [10]. We retrospectively reviewed the clinical characteristics of 200 cone biopsies and compared the results of cytological LSILs according to the HPV test findings.
Two hundred cases of cone biopsy results at Hallym University Sacred Heart Hospital from February 2006 to February 2008 were reviewed retrospectively. The mean age was 42.3±9.24 (range, 22-80) years.
During the review of pathologic reports, including punch and cone biopsies, severe reports were selected if two categories were presented in biopsies. There were 21 cervicitis (10.5%), 59 LSILs (29.5%), 99 HSILs (49.5%), and 21 cancers (10.5%). There were 26 pathological LSIL patients preoperatively. HPV typing with DNA microarrays (Biomedlab, Seoul, Korea) were performed in 20 patients preoperatively in LSIL patients. Available high risk type HPVs were 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, and 69. Low risk type HPVs were 6, 11, 34, 40, 42, 43, 44 in our microarray kits. The HRT HPV positive results of DNA microarrays were 16 patients out of 20 patients with cytological LSIL.
Chi-square and student-t tests were used in analysis. Results with P-value less than 0.05 were regarded as statistically significant.
Pathologic diagnoses and HRT-HPV positivities were 78.9% (n=30) for LSIL, 74.7% (n=59) for HSIL (CIN 2, 3, CIS) and 76.9% (n=10) for cancer patients (P=0.877).
In the LSIL patients, HRT-HPV positive patients were 16/20 (80%) and one LRT-HPV positive patient was 1/20 (5%). This means that 75% of LSIL patients have HRT-HPV in their lesions. A further indication is that 75% of LSIL may develop into HSIL or cancer. Low risk HPV was only found in LSIL.
Among the LSIL patients CIN 1 were 16/26 (61.5%), CIN 2 were 2/26 (7.7%), CIN3 were 5/26 (19.2%), CIS was 1/26 (3.8%), severe than CIN2 were 8/26 (30.8%) and chronic cervicitis were 2 patients (7.7%). The concordance rate between cytology and pathology in LSIL was 61.5%.
There were 26 cytological LSIL patients, and HPV test results were found in 20 of them. One low risk HPV positive patient 5% (1/20) was pathologically CIN 1. Sixteen (16/20) 80% of the cytologic LSIL patients were positive in HRT-HPVs (Fig. 5A).
In HRT-HPV positive cytologic LSIL 16 patients, 6/16 (37.5%) were more severe than HSIL upon pathologic diagnoses. The HSIL incidence was higher than the HRT-HPV negative group 2/10 (20%) and overall 8/26 (30.8%), but the rate of incidence has no statistical significance in our study (P=0.42) (Fig. 5B).
In the management of cervical intraepithelial lesions, HPV tests are the preferred method of clinical aid. In cytological LSIL patients, we can do colposcopy directed punch biopsy and an HPV test in order to confirm diagnosis and clinical guidance.
In Anyang city, cone biopsies are commonly performed under the diagnosis of HSIL from the ages of 30-40 years. In ALTS, 83% of the women of the LSIL group were positive for high-risk type HPVs [11]. HRT-HPV prevalence was similar in pathological LSILs (80%) and HSILs (81.6%) patients in our study (Table 3). The prevalence of HRT HPVs were similar between ALTS group study and our study. This result confirms that LSIL is a very specific indicator of the presence of HRT-HPV. LSIL is a disease induced by HPVs.
The life of LSIL is not as serious. 90% of adolescent LSIL will spontaneously regress (< 21 years of age) [12,13]. The HSIL rate is lower in post-menopause cytological LSIL women and they can be managed in the same way as ASC-US management in post-menopause [14,15]. 90% of high risk HPV positive cytological LSIL patients spontaneously regress within 24 months [16]. 70% of LSIL with high risk HPV infected patients were spontaneously cleared of their infections [17]. Therefore, surgical treatment of LSIL is usually not preferred.
However, it is necessary for pathological LSIL patients whose history was preceded by cytological HSIL or atypical glandular cells to undergo diagnostic excision biopsies or 6 month interval follow ups of colposcopy and cytology [10]. HRT HPV negative LSILs and low risk type HPV positive LSILs were found to be pathologically benign, as we expected. And a trend appears to exist in that high risk HPV positive cytological LSIL patients have increased rates of pathologically HSIL and cancer. It is not such a small portion as to be ignored and reaches approximately 30% [17]. Although our study has limited statistical influence, further research in HRT HPV positive LSIL patients would seem to be required.
Cytological LSIL women with high risk type HPV should be managed cautiously, with cytology and colposcopy, and by at least a 6 month interval follow-up. Histologic diagnosis by punch biopsy or excision is also recommended in HRT HPV positive LSILs. A large scale study in LSIL patients would seem to be mandated as being necessary in relation to high risk type HPVs.
Figures and Tables
Fig. 1
The age and pathologic distribution of cone biopsies. HSILs are most frequent in the fifth decade. LSIL, low grade squamous intraepithelial lesion; HSIL, high grade squamous intraepithelial lesion.

Fig. 2
High risk HPV type positivity in all pathologic diagnosis of cone biopsies. High risk type HPVs are similar in prevalence among LSIL (78.9%), HSIL (74.7%) and cancer (76.9%) groups (P=0.877). LSIL, low grade squamous intraepithelial lesion, HSIL, high grade squamous intraepithelial lesion; HPV, human papillomavirus.
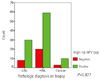
Fig. 3
High risk HPV positivity according to the cytologic diagnoses of patients. LSIL, low grade squamous intraepithelial lesion; HSIL, high grade squamous intraepithelial lesion; ASCUS, atypical squamous cells of undetermined significance; ASC-H, atypical squamous cells cannot exclude HSIL; HPV, human papillomavirus.

Fig. 4
Histologic diagnoses in cytologic LSIL patients. The cytologic LSILs were pathological LSIL in biopsies (61.5%). The cytologic LSILs were histologic HSIL in 8/26 (30.7%). Vertical axis represents ratio (%) of every categories in cytologic LSILs. LSIL, low grade squamous intraepithelial lesion; HSIL, high grade squamous intraepithelial lesion; CIN, cervical intraepithelial lesion; CIS, carcinoma in situ.

Fig. 5
High risk type HPVs in cytological LSILs are probably related to increasing histological HSIL. (A) 80% of high risk HPV positive cytologic LSILs were histologic HSIL. 5% of low risk HPV positive cytologic LSILs were histologic HSIL. The vertical axis represents ratio of each categories in LSIL patients. (B) High risk HPV positive cytologic LSIL patients were higher than other groups-over all cytologic LSIL and high risk HPV negative cytologic LSIL patients. The vertical axis represents ration of every categories in LSIL patients. LSIL, low grade squamous intraepithelial lesion; HPV, human papillomavirus.

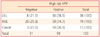
Table 3
High risk HPV positivity according to the cytologic diagnoses of patients

The prevalence of high risk HPVs are similar in HSIL (80%) and LSIL (81.6%).
HPV, human papillomavirus; LSIL, low grade squamous intraepithelial lesion; HSIL, high grade squamous intraepithelial lesion; ASCUS, atypical squamous cells of undetermined significance; ASC-H, atypical squamous cells cannot exclude HSIL.
References
1. Sulik SM, Kroeger K, Schultz JK, Brown JL, Becker LA, Grant WD. Are fluid-based cytologies superior to the conventional Papanicolaou test? A systematic review. J Fam Pract. 2001. 50:1040–1046.
2. Taylor S, Kuhn L, Dupree W, Denny L, De Souza M, Wright TC Jr. Direct comparison of liquid-based and conventional cytology in a South African screening trial. Int J Cancer. 2006. 118:957–962.
3. Ronco G, Segnan N, Giorgi-Rossi P, Zappa M, Casadei GP, Carozzi F, et al. Human papillomavirus testing and liquid-based cytology: results at recruitment from the new technologies for cervical cancer randomized controlled trial. J Natl Cancer Inst. 2006. 98:765–774.
4. Mitchell MF, Tortolero-Luna G, Wright T, Sarkar A, Richards-Kortum R, Hong WK, et al. Cervical human papillomavirus infection and intraepithelial neoplasia: a review. J Natl Cancer Inst Monogr. 1996. 17–25.
5. ter Haar-van Eck SA, Rischen-Vos J, Chadha-Ajwani S, Huikeshoven FJ. The incidence of cervical intraepithelial neoplasia among women with renal transplant in relation to cyclosporine. Br J Obstet Gynaecol. 1995. 102:58–61.
6. ter Harmsel B, Smedts F, Kuijpers J, van Muyden R, Oosterhuis W, Quint W. Relationship between human papillomavirus type 16 in the cervix and intraepithelial neoplasia. Obstet Gynecol. 1999. 93:46–50.
7. Solomon D, Schiffman M, Tarone R. ALTS Study group. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst. 2001. 93:293–299.
8. Cox JT, Schiffman M, Solomon D. ASCUS-LSIL Triage Study (ALTS) Group. Prospective follow-up suggests similar risk of subsequent cervical intraepithelial neoplasia grade 2 or 3 among women with cervical intraepithelial neoplasia grade 1 or negative colposcopy and directed biopsy. Am J Obstet Gynecol. 2003. 188:1406–1412.
9. Park TW, Fujiwara H, Wright TC. Molecular biology of cervical cancer and its precursors. Cancer. 1995. 76:1902–1913.
10. Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol. 2007. 197:340–345.
11. The Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS) Group. Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intraepithelial lesions: baseline data from a randomized trial. J Natl Cancer Inst. 2000. 92:397–402.
12. Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chapter 5: Updating the natural history of HPV and anogenital cancer. Vaccine. 2006. 24:Suppl 3. S342–51.
13. Moscicki AB, Hills N, Shiboski S, Powell K, Jay N, Hanson E, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. 2001. 285:2995–3002.
14. Sherman ME, Schiffman M, Cox JT. Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesion Triage Study Group. Effects of age and human papilloma viral load on colposcopy triage: data from the randomized Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesion Triage Study (ALTS). J Natl Cancer Inst. 2002. 94:102–107.
15. Evans MF, Adamson CS, Papillo JL, St John TL, Leiman G, Cooper K. Distribution of human papillomavirus types in ThinPrep Papanicolaou tests classified according to the Bethesda 2001 terminology and correlations with patient age and biopsy outcomes. Cancer. 2006. 106:1054–1064.
16. Schlecht NF, Platt RW, Duarte-Franco E, Costa MC, Sobrinho JP, Prado JC, et al. Human papillomavirus infection and time to progression and regression of cervical intraepithelial neoplasia. J Natl Cancer Inst. 2003. 95:1336–1343.
17. Nobbenhuis MA, Helmerhorst TJ, van den Brule AJ, Rozendaal L, Voorhorst FJ, Bezemer PD, et al. Cytological regression and clearance of high-risk human papillomavirus in women with an abnormal cervical smear. Lancet. 2001. 358:1782–1783.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download