Abstract
Objective
To evaluate the therapeutic efficacy of dilatation and evacuation (D&E) for cesarean scar pregnancy (CSP) and to determine prognostic factors.
Methods
This study was retrospectively performed in 68 women who had been diagnosed with CSP. The 56 CSP women were enrolled for whom D&E was the primary treatment. The patients were divided into two groups according to treatment outcomes. Women who received D&E only were defined as the success group and patients who required additional treatment were categorized as the failure group. Demographic, clinical, and sonographic characteristics according to treatment results were analyzed.
Results
Among the total of 56, 36 (64%) women were treated by D&E only, and 20 (36%) women underwent additional treatments. There were no significant difference in maternal age, gravida, number of previous cesarean section, initial symptoms, but significant difference in parity (≥2), gestational age at diagnosis, serum β-human chorionic gonadotropin level, postoperative stay, and time to resolution was present (P <0.05). On sonographic findings, there was significant difference in terms of fetal heart beat, hematoma and myometrial thickness of the implantation site (P <0.05). After multivariate regression, only myometrial thickness of the implantation site with a cut-off value of 1.75 mm was significant.
Cesarean scar pregnancy is the rarest form among ectopic pregnancies, but its frequency is increasing due to recent increase in cesarean deliveries. Early diagnosis of cesarean scar pregnancy is most important, for it can give rise to grave complications such as uterine rupture or massive hemorrhaging, and thus have an effect on maternal morbidity or mortality rate [1].
Total hysterectomy has been a common treatment modality since this form of pregnancy has shown cases with initial manifestations of massive hemorrhage as an obstetrical emergency [2]. With advances in ultrasonography resolution, detection at an earlier gestational age and more accurate diagnosis became possible to enable various methods for preservation of fertility, such as systemic or local administration of Methotrexate® (MTX, Hospital Korea, Seoul, Korea) into the cesarean scar, hemostasis using balloon catheter after ultrasound-guided dilatation and curettage, laparoscopic or hysteroscopic guided wedge excision, and bilateral uterine artery embolization therapy [3]. However cesarean scar pregnancy cases are still low in numbers, and the guideline on treatment modality is not determined yet through large scale studies, with much refute in its selection consensus [4,5]. Invasive methods, such as dilatation and evacuation (D&E), have especially low success rates and high complication rates, such as intraoperative or postoperative massive bleeding [6,7]. But as more and more cesarean scar pregnancies are experienced and treated, the authors have come to select D&E as the primary treatment modality, since this modality entails shorter hospitalization and treatment period, as well as lower complication rate than that of prior studies. Therefore this study aims to ascertain out the therapeutic efficacy of D&E, and to determine the prognostic factors of 56 patients who have been diagnosed with cesarean scar pregnancy who received D&E at our center.
The medical records of 68 patients diagnosed with cesarean scar pregnancy from June, 2000 to June, 2009 at Ajou University Medical Center were reviewed retrospectively. The 12 patients with medical treatment using MTX, or surgery as the primary treatment were excluded. The total of 56 patients who received ultrasound-guided D&E as the primary treatment were selected in the study analysis. The 36 patients who completely recovered with only D&E were categorized as the success group, and the 20 patients who received additional treatment such as insertion of 23-Fr cervical Foley catheter for vaginal bleeding after the D&E (n=7), or chemotherapy with supplementary MTX (n=4), or hysterectomy (n=1), or combined therapy (n=8) were categorized as the treatment failure group. Cervical tamponade was employed in patients with immediate bleeding episode during or after evacuation. Chemotherapy was administered those with increased or plateau serum β-human chorionic gonadotropin (β-hCG) levels. Combination therapy is defined as cervical tamponade plus additional systematic MTX administration. One case of hysterectomy was carried out in an unstable hemodynamic status during evacuation due to massive bleeding at 8th gestational weeks. Four cases in the success group and nine cases in the failure group required transfusions.
Before choosing a treatment method among the above therapeutic options, we fully discussed with the patients and their families about the risks of each treatment modality (including exploratory laparotomy, laparoscopy, surgical evacuation, and medical therapy) associated with cesarean scar pregnancy. Written informed consent was obtained in all cases. This study was approved by the Institutional Review Board and Ethics Committee (IRB:AJIRB-MED-MDB-11-042).
The ultrasonographic diagnosis of cesarean scar pregnancy is defined as the presence of; 1) empty uterine cavity and cervical canal, 2) gestational sac on the anterior isthmic portion and the presence of myometrial defect between bladder and gestational sac, 3) discontinuity in uterine anterior wall on sagittal plane, 4) negative sliding organ sign. All these cases had a connection between the endometrial cavity and gestational sac, and no intramural type was present among the cases enrolled (Fig. 1). Follow-up was every two weeks after discharge, and complete recovery was determined at the outpatient care after surgery, with no residual tissue of the scar as scanned by ultrasonography, and with normal serum β-hCG levels.
In both groups, predictive factors of D&E were evaluated through correlation between demographic factors including maternal age, obstetric history, prior cesarean section numbers, gestational weeks at diagnosis, initial serum β-hCG level, clinical manifestations at diagnosis, hospitalization stay, recovery period after the operation, and ultrasonographic manifestations such as the fetal heart beat, hematoma, and the myometrial thickness on the implantation location at the time of diagnosis, and treatment outcomes. The gestational age at diagnosis was estimated using menstrual history. In uncertain cases, the gestational age was modified using ultrasonographic findings such as gestational sac, and/or crown rump length.
The cut-off level of significant parameters was determined through the receiver-operator curve, and the odds ratio was calculated through multivariate regression.
For statistical analysis, SPSS ver 12.0 (SPSS Inc., Chicago, IL, USA), chi-square, Mann-Whitney U test and logistic regression were used, and P-value less than 0.05 was defined as statistically significant.
Among 68 patients who were diagnosed with cesarean scar pregnancy, 56 patients were enrolled in this study; 36 (64%) patients received successful treatment with only D&E, and 20 (36%) patients received additional treatment. No statistically significant difference were observed when comparing the success and failure group with regard to maternal age, gravid, and prior cesarean section numbers had. Only parity (≥2) was of statistical significance. Both the success and failure group showed no signigicance symptoms or vaginal bleeding as the initial clinical manifestation, and there was no correlation between the two groups with regard to quantity of bleeding. The serum β-hCG levels and gestational weeks at the time of diagnosis were significantly lower in the success group, and also the patients were diagnosed earlier. The hospital stay was 4.7 ± 2.0 days in failure group, which was significantly longer than the success group with 2.2 ± 0.4 days. Complete time to recovery was 20.1 ± 9.2 days, which was significantly shorter in the success group compared to the failure group with 35.7 ± 20.7 days (Table 1).
Comparing the ultrasound findings in the group that received only D&E procedure, the procedure alone was inadequate for complete treatment when fetal heart beat or a hematoma was present at the time of the diagnosis, and eventually complications occurred. The myometrial thickness (mm) of the implantation site between the gestational sac and bladder serosa was 2.6 ± 0.7 in the single procedure group, and in the additional treatment group it was 1.2 ± 1.0, which showed that myometrial thickness was significantly increased in the success group (Table 2).
The cut-off value of each significant clinical and sonographic parameters using receiver-operator curve were 5.7 gestational weeks at the time of diagnosis, serum β-hCG level < 30,000 mIU/mL at the time of diagnosis, and myometrial thickness > 1.75 mm on implantation site (Figs. 2, 3, 4, respectively). But when each of the parameters were statistically analyzed with multiple logistic regression, myometrial thickness >1.75 mm of implantation site was the only significant factor in determining the treatment outcome with D&E (Table 3).
Cesarean scar pregnancy is the rarest form of ectopic pregnancy, with a frequency of 1 out of 1,800 to 2,216 pregnancies among women who have received at least one prior cesarean section [6,8]. Since all cases in this study were referral cases, determining the actual frequency is very difficult. The reason that our institution has a large number of cases is because we receive many referral cases from primary and secondary clinics as we are a tertiary center since the late 90's after introduction and diagnosis of first case cesarean scar pregnancy. In 1978, Larsen and Solomon [9] presented a case report and later on, Fylstra [4] reported a review article organizing 18 cases. The incidence of cesarean scar pregnancy is increasing with awareness and advances in ultrasonography resolution.
Diagnosis is usually achieved by transvaginal ultrasonography, and differential diagnosis from progressive spontaneous abortion and cervico-isthmic ectopic pregnancy is important, since there are marked differences not only in the treatment but also in the prognosis [10]. Maymon et al. [11] pointed out the importance of transabdominal sonography with a full bladder to acquire 'panoramic view' images, and also in our experience, observation of the bladder interface and the gestational sac within the myometrium was especially informative. Thus, combined sonography is recommended when a cesarean scar pregnancy is suspected.
Since cesarean scar pregnancy is rare, studies are usually case reports or small case series, and the consensus for treatment modality are not yet established. As the gestation period progresses, serious obstetrical complications such as uterine rupture and massive bleeding which increases the maternal mortality and morbidity can occur. Considering this in general, cessation of pregnancy is induced, and the treatment modalities can be divided broadly into two paths, which are medical and surgical treatments.
Medical treatments are, as in ectopic tubal pregnancy, administration of MTX systemically [6,12,13], or local injection within the amniotic cavity [8,10,14], combination therapy into both routes [5,6], and administration of MTX into the amniotic cavity after embryocide using potassium chloride if there is fetal heart beat [8]. There is no selection guideline for systemic or local injection, but systemic administration was reported to be effective when the serum β-hCG level is less than 5,000 IU/mL [6]. Because of the relatively short half life of MTX, local injections are predicted to decrease the drug side effects that occurs after systemic administration, as well as maintaining the high MTX concentration within the amniotic cavity to increase treatment success rate, but more fundamental studies on the actual serum drug level is needed. In fact, in selecting between conservative medical and surgical treatment, Jurkovic et al. [8] suggested that MTX local injection be used if the gestational age is greater than 7 weeks and surgical evacuation be used if medical therapy failed. Also, Maymon et al. [5] suggested conservative medical treatment for the followings criteria: pain free, hemodynamically stable, unruptured state at less than 8 weeks, and myometrial thickness < 2 mm at implantation site.
Trials of D&E, which is a surgical treatment modality, has been very disappointing, as in one review, it was said to be suboptimal and risky [3]. But in case series with more than two cases, one study including 8 cases; four cases of gestational age at 4-6 weeks and one case of gestational age at 10 weeks, were treated just with D&E without additional treatment. In the remaining three cases of 11, 14, and 23 gestational weeks, foley catheter was used for the compression of hemorrhage [8]. Arslan et al. [15] added a successful case at 7th gestational weeks with suction curettage. Wang and Tseng [7], who experienced 3 cases, reported that the two cases which received surgery each at 4th and 5th weeks suffered no complications, and one case that received surgery at 7th week needed additional laparoscopic operation due to hemorrhage. Though the classification is different, in a larger study, Hwang et al. [16] reported better success rates with D&E or D&E under laparoscopic guide. Also compared to 46% of success rate in D&E treatment group, our report showed more increased success rate with 64% [17]. Taken together, for successful evacuation, surgery at earlier gestational weeks is one of the determining prognostic factors.
The other problem is locally injected MTX, where the serum β-hCG level normalization time is so long such that there are reports of a few weeks, months, and even one year. Even if the serum β-hCG level decreases after MTX administration, the lesion is reported to become larger on follow-up ultrasonography, which renders it difficult to decide whether additional treatment is needed. Late hemorrhage after administration have been reported [18]. A very important advantage of dilatation and curettage is that it requires less hospitalization stay. The first case the authors experienced was at 6 gestational weeks, and very careful evacuation under transabdominal ultrasound guidance was satisfactory, so that dilatation and curettage was selected as the primary treatment modality in many later cases.
The most important prognostic factor is degree of myometrial invasion. Wang and Tseng [7] explained that deep implantation was the reason for an alternative treatment other than evacuation, in treating a cesarean scar pregnancy at 7.2 gestational weeks. They presented a bulging gestational sac seen at ultrasonography as an evidence of deep implantation. However, if the degree of implantation could be quantitative using sonographic measurement of myometrial thickness at the implantation site in cesarean scar pregnancy with a connection between the endometrial cavity and gestational sac, anterior myometrial thickness of the implantation site may be an useful objective method for estimating myometrial invasion with clinical significance for prognostic factors. Anterior myometrial thickness at the implantation site presented at this study is a significant predictive factor of curettage, and when the cut-off value is less than 1.75 mm, other additional treatment approach is needed.
In conclusion, cesarean scar pregnancy is rare but a life-threatening condition without a treatment guideline to date. Due to the small number of studies, experience with dilatation and curettage as a surgical treatment modality is disappointing. But if D&E is carried out early in the gestation together with early diagnosis using ultrasonography, under adequate guidelines in selected cases with the expertise of an obstetrician, it may become an excellent primary treatment modality for cesarean scar pregnancy.
Figures and Tables
Fig. 1
Sagittal transvaginal image showing a gestational sac located at the scar site and thin myometrial thickness (two white arrows) at the implantation site (white arrowheads: endometrial lining, *: internal os).
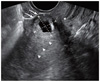
Fig. 2
Receiver operating characteristic curve of gestational age at diagnosis (area under curve=0.762, P=0.001, 95% confidence interval [0.62-0.90]). The cut-off point is 5.7 weeks (sensitivity=0.85; 1-specificity= 0.45).
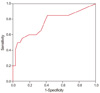
Fig. 3
Receiver operating characteristic curve of initial β-human chorionic gonadotropin level (area under curve=0.852, P<0.001, 95% confidence interval [0.74-0.97]). The cut-off point is 30,000 mIU/mL (sensitivity=0.90; 1-specificity=0.32).
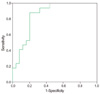
Fig. 4
Receiver operating characteristic curve of myometrial thickness at the implantation site (area under curve=0.837, P<0.001, 95% confidence interval [0.71-0.96]). The cut-off point is 1.75 mm (sensitivity=0.90; 1-specificity=0.20).

References
1. Coniglio C, Dickinson JE. Pregnancy following prior Caesarean scar pregnancy rupture: Lessons for modern obstetric practice. Aust N Z J Obstet Gynaecol. 2004. 44:162–165.
2. Marcus S, Cheng E, Goff B. Extrauterine pregnancy resulting from early uterine rupture. Obstet Gynecol. 1999. 94:804–805.
3. Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006. 107:1373–1381.
4. Fylstra DL. Ectopic pregnancy within a cesarean scar: a review. Obstet Gynecol Surv. 2002. 57:537–543.
5. Maymon R, Halperin R, Mendlovic S, Schneider D, Vaknin Z, Herman A, et al. Ectopic pregnancies in Caesarean section scars: the 8 year experience of one medical centre. Hum Reprod. 2004. 19:278–284.
6. Seow KM, Huang LW, Lin YH, Lin MY, Tsai YL, Hwang JL. Cesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol. 2004. 23:247–253.
7. Wang CB, Tseng CJ. Primary evacuation therapy for Cesarean scar pregnancy: three new cases and review. Ultrasound Obstet Gynecol. 2006. 27:222–226.
8. Jurkovic D, Hillaby K, Woelfer B, Lawrence A, Salim R, Elson CJ. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scar. Ultrasound Obstet Gynecol. 2003. 21:220–227.
9. Larsen JV, Solomon MH. Pregnancy in a uterine scar sacculus--an unusual cause of postabortal haemorrhage. A case report. S Afr Med J. 1978. 53:142–143.
10. Godin PA, Bassil S, Donnez J. An ectopic pregnancy developing in a previous caesarian section scar. Fertil Steril. 1997. 67:398–400.
11. Maymon R, Halperin R, Mendlovic S, Schneider D, Herman A. Ectopic pregnancies in a Caesarean scar: review of the medical approach to an iatrogenic complication. Hum Reprod Update. 2004. 10:515–523.
12. Weimin W, Wenqing L. Effect of early pregnancy on a previous lower segment cesarean section scar. Int J Gynaecol Obstet. 2002. 77:201–207.
13. Lam PM, Lo KW, Lau TK. Unsuccessful medical treatment of cesarean scar ectopic pregnancy with systemic methotrexate: a report of two cases. Acta Obstet Gynecol Scand. 2004. 83:108–111.
14. Haimov-Kochman R, Sciaky-Tamir Y, Yanai N, Yagel S. Conservative management of two ectopic pregnancies implanted in previous uterine scars. Ultrasound Obstet Gynecol. 2002. 19:616–619.
15. Arslan M, Pata O, Dilek TU, Aktas A, Aban M, Dilek S. Treatment of viable cesarean scar ectopic pregnancy with suction curettage. Int J Gynaecol Obstet. 2005. 89:163–166.
16. Hwang JH, Lee JK, Oh MJ, Lee NW, Hur JY, Lee KW. Classification and management of cervical ectopic pregnancies: experience at a single institution. J Reprod Med. 2010. 55:469–476.
17. Sadeghi H, Rutherford T, Rackow BW, Campbell KH, Duzyj CM, Guess MK, et al. Cesarean scar ectopic pregnancy: case series and review of the literature. Am J Perinatol. 2010. 27:111–120.
18. Lai YM, Lee JD, Lee CL, Chen TC, Soong YK. An ectopic pregnancy embedded in the myometrium of a previous cesarean section scar. Acta Obstet Gynecol Scand. 1995. 74:573–576.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download