Abstract
The incidence of endometrial cancer (EC) has increased and it has approximately 16% of gynecologic cancers in Korea. For loco-regional or distant recurrence, adjuvant treatment after surgery is very important in patients with intermediate- or high-risk EC (IHR-EC). Recently clinical trials for treating patients with IHR-EC are focused on the efficacy of concurrent chemoradiation (CCR). However, increase in intestinal complications in the group of radiotherapy combined with chemotherapy was reported. In case of chemoradiation-related superior mesenteric artery (SMA) occlusion, it could be fatal nevertheless with intensive care. We present a case report of radiation-related arteritis leading to stenosis in SMA, which led the patient with EC to death in a year after CCR.
Endometrial cancer (EC) is the most common gynecologic malignancy in the USA, and its incidence has increased to approximately 16% of gynecologic cancers in Korea [1]. More than 80% of patients have International Federation of Gynecology and Obstetrics stages I or II of the disease [2]. Although most patients with low-risk EC may be cured after surgery, those with intermediate- or high-risk endometrial cancer (IHR-EC) often show loco-regional or distant recurrence [3]. Therefore, adjuvant treatment after surgery is very important in patients with IHR- EC.
Nowadays clinical trials for treating patients with IHR-EC are focused on the efficacy of concurrent chemoradiation (CCR) after a phase II study of the Radiation Therapy Oncology Group (RTOG 9708), which demonstrated that CCR may improve loco-regional control, and reduce extra-pelvic recurrence [4-6]. By the way, radiation-related arteritis (RRA) is a rare but well recognized complication of radiotherapy. A study reported increase in intestinal complications in the group of radiotherapy combined with chemotherapy [7].
We present a case report of RRA leading to stenosis in the superior mesenteric artery (SMA), which led the patient with EC to death in a year after CCR.
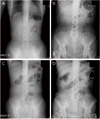
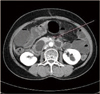
A 62-year-old woman visited her physician complaining of vaginal spotting which started 5 years ago. She was transferred to our institution under the impression of EC. Endometrial biopsy revealed endometrioid EC (EEC), grade 1. Preoperative computed tomography (CT) implied EC without parametrial invasion or cervical invasion, and showed neither distant metastasis nor lymphadenopathy. CA-125 level was elevated to 364.95 U/mL. A total hysterectomy, both salpingo-oophorectomies (TH+BSO), pelvic and para-aortic lymphadenectomy was done as cancer cells were found at paraaortic lymph nodes excised during operation. Permanent biopsy reported EEC grade 1, and the size of the tumor was 4.5 × 3.5 cm, and the cancer had metastasized to 13 out of 42 regional lymph nodes (LN) including paraaortic LN (Fig. 1). CCR was applied using doxorubicin and cisplatin for 2 times, and pelvis radiation using 10MV energy with total dose 3,240 cGy, daily tumor dose 180 cGy, 18 fractions for 5 weeks simultaneously. One day she came to emergency room for acute and severe abdominal pain. X-ray films showed normal abdominal finding with multiple surgical clips (Fig. 2A, 2B). The next day, pain did not subside. Abdominal CT showed small bowel ischemia with SMA thrombosis (Fig. 3). Just before operation, simple X-ray hinted at ileus with diffuse edematous bowel wall thickening (Fig. 2C, 2D). Emergent operation of nearly total small bowel resection was done. The pathologic report revealed thrombus with intimal fibrinous necrosis and fibrosis (Fig. 4). After operation, general care has been given. Complications related to central and peripheral nutrition, stress ulcer as well as recurrent infection for a long time had induced her emaciated. Even though intensive care had been given, she died after 1 year.
Radiation therapy (RT) is the most effective adjuvant treatment in patients with IHR-EC, and many gynecologic oncologists prefer RT for treating patients after surgery [8]. Patients with stage II and III disease are also offered postoperative radiotherapy. There have been three randomised trials that have addressed the effect of postoperative radiotherapy in high-risk patients [8,9]. No overall survival (OS) advantage has ever been shown for postoperative radiotherapy, but an improved progression-free survival (PFS) of around 10% has been seen.
Doxorubicin is one of the most effective agents in the treatment of EC, and doxorubicin/cisplatin (AP) is in most countries commonly accepted as the standard regimen for EC [10]. Currently, chemoradiation is not regarded as standard management for EC. The clinical trial data in this area are sparse [11]. The Gynecologic Oncology Group study (GOG-122) randomised 422 patients with stage III or IV EC after TH+BSO and maximal debulking with no residual tumour less than 2 cm into radiotherapy consisting of whole abdominal radiotherapy delivering 30 Gy in 20 fractions followed by an additional 15 Gy pelvic boost and chemotherapy in which AP were given every 3 weeks for seven cycles followed by one cycle of cisplatin. Both OS and PFS were significantly better for patients receiving chemotherapy, despite an increased incidence of grade 3 and 4 toxicity, such that less than two-thirds of patients completed all eight cycles of chemotherapy [12]. A study randomised 156 patients with high-grade disease or stage IC-IIIA disease to receive radiotherapy alone or radiotherapy combined with three courses of chemotherapy. No difference in OS or PFS was seen in this study either and there was an increase in intestinal complications in the chemotherapy group [7]. However, there is increasing interest in the role of chemotherapy in high-risk EC and this is the basis of the current PORTEC-3 study. This study randomized patients with stage I or IIA grade 3 disease and stages IIB and above after hysterectomy to receive either radiotherapy alone or chemoradiation with two courses of cisplatin week 1 and week 4 followed by adjuvant chemotherapy with carboplatin and docetaxel.
RRA has since been described in numerous large vessels [13]. These lesions include fibrosis of the internal elastic membrane, injury to the vasa vasorum, and ischemic necrosis of the vessel wall, periarterial fibrosis, and hyalinization and thickening of the vessel wall with fibrin deposition [14]. The dose of radiation associated with this unique clinical entity is 20 to 80 Gy [14], and 39.5 to 80 Gy is specifically mentioned for the iliac and femoral arteries [13]. The dose used to the patient was much higher than these.
Treatment of RRA has not been extensively studied, likely because of the scarcity of cases. The basic treatment options described in the literature include medical therapy, anatomic revascularization, extraanatomic bypass, percutaneous transluminal angioplasty, and stenting (balloon-expandable and self-expanding stents) [15].
Although RRA in the large vessels is a rare complication of radiotherapy, it may become more common as a result of the increasing use of radiotherapy and increasing duration of survival in patients with cancer. We tried to review the risk factors for SMA embolisation after CCR, but we could not find these. We present a case report of RRA leading to stenosis in SMA, which leaded the patient with EC to death in a year after CCR.
Figures and Tables
Fig. 1
This slide shows (A) endometrial cancer grade 1 (H&E, ×100), and (B) positive lymph node metastases (H&E, ×20).

References
1. Lee HP. Annual report of gynecologic cancer registry program in Korea: 1991~2004. Korean J Obstet Gynecol. 2008. 51:1411–1420.
2. Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006. 95:Suppl 1. S105–S143.
3. Kuwabara Y, Susumu N, Banno K, Hirao T, Kawaguchi M, Yamagami W, et al. Clinical characteristics of prognostic factors in poorly differentiated (G3) endometrioid adenocarcinoma in Japan. Jpn J Clin Oncol. 2005. 35:23–27.
4. Greven K, Winter K, Underhill K, Fontenesci J, Cooper J, Burke T. Final analysis of RTOG 9708: adjuvant postoperative irradiation combined with cisplatin/paclitaxel chemotherapy following surgery for patients with high-risk endometrial cancer. Gynecol Oncol. 2006. 103:155–159.
5. CliniclaTrials.gov. Comparison of radiation therapy with or without combination chemotherapy following surgery in treating patients with stage I or stage II endometrial cancer [Internet]. 2008. cited 2008 Nov 12. Bethesda (MD): National Library of Medicine (US);Available from:
http://clinicaltrials.gov/ct2/show/NCT00006027?%20term=RTOG+9905&rank=1.
6. ClinicalTrials.gov. Chemotherapy and radiation therapy compared with radiation therapy alone in treating patients with high risk stage I, stage II, or stage III endometrial cancer [Internet]. 2008. cited 2008 Nov 12. Bethesda (MD): National Library of Medicine (US);Available from:
http://clinicaltrials.gov/ct2/show/NCT00411138?term=PORTEC-3&rank=1.
7. Kuoppala T, Mäenpää J, Tomas E, Puistola U, Salmi T, Grenman S, et al. Surgically staged high-risk endometrial cancer: randomized study of adjuvant radiotherapy alone vs. sequential chemo-radiotherapy. Gynecol Oncol. 2008. 110:190–195.
8. Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám-Rodenhuis CC, et al. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. Lancet. 2000. 355:1404–1411.
9. ASTEC/EN.5 Study Group. Blake P, Swart AM, Orton J, Kitchener H, Whelan T, Lukka H, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet. 2009. 373:137–146.
10. Kim JH, Lee SJ, Bae JH, Lee SH, Bae SN, Namkoong SE, et al. Adjuvant therapy in high-risk early endometrial carcinoma: a retrospective analysis of 46 cases. J Gynecol Oncol. 2008. 19:236–240.
11. Hogberg T. Adjuvant chemotherapy in endometrial carcinoma: overview of randomised trials. Clin Oncol (R Coll Radiol). 2008. 20:463–469.
12. Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006. 24:36–44.
13. Andros G, Schneider PA, Harris RW, Dulawa LB, Oblath RW, Salles-Cunha SX. Management of arterial occlusive disease following radiation therapy. Cardiovasc Surg. 1996. 4:135–142.
14. Chuang VP. Radiation-induced arteritis. Semin Roentgenol. 1994. 29:64–69.
15. Ting AC, Cheng SW, Yeung KM, Cheng PW, Lui WM, Ho P, et al. Carotid stenting for radiation-induced extracranial carotid artery occlusive disease: efficacy and midterm outcomes. J Endovasc Ther. 2004. 11:53–59.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download