Abstract
The occurrence of malignant pericardial effusion is rare in squamous cell carcinoma of the uterine cervix. Only six cases of antemortem diagnosis have been reported. We report a patient with squamous cell carcinoma of the cervix who received emergent pericardiocentesis. In a few reported cases, women with pericardial involvement are candidates for radiation or chemotherapy. Early detection and prompt management of pericardial effusion is correlated with better quality of life and a decreased morbidity.
Cervical cancer is the second most common cancer in women worldwide, and nearly a third of patients who present with invasive cervical cancer will die of this disease [1]. Metastasis of squamous cell carcinoma of the uterine cervix to the pericardium is an uncommonly appreciated event. Tumors often metastasize to the pericardium in patients with melanoma, lung carcinoma, lymphoma, and breast carcinoma and sarcoma [2]. The incidence of cardiac metastasis determined by autopsy data has been reported to be 0.3-3.2% in deceased patients with cervical cancer [3]. According to a review of the literature, only six previously reported cases of antemortem diagnosis of malignant pericardial effusion from carcinoma of the cervix have been presented in detail [4]. We report a patient with squamous cell carcinoma of the cervix who received emergent pericardiocentesis.
A 52-year-old, multiparous (para 1-0-0-1) woman was referred to our hospital in June 2009 with a one-month history of dyspnea and cough. She had a negative personal and family history for other diseases. The cytology of pleural effusion was negative for malignancy. She had a palpable mass on the left side of the neck. The examination of her left neck lymph nodes revealed squamous cell carcinoma, and her positron-emission tomography-computed tomography (PET-CT) scan showed both hydronephrosis, hypermetabolism of the uterus, and metastatic lymphadenopathies at the perigastric, peripancreatic, para-aortic, left axillary, both supraclavicular, and the left neck level II-V areas.
On pelvic examination, the cervical tumor was found to involve the vagina and the parametrium. Cervical biopsy showed squamous cell carcinoma. From June 2009 to August 2009, the patient received three cycles of 5-FU and cisplatin chemotherapy and concurrent radiation. After chemo-radiotherapy, follow-up PET-CT showed no evidence of hypermetabolism at the cervix, but metastatic lymphadenopathy was remained in the right inguinal area. However, all results showed an improved state since 22 June 2009. PET-CT showed stable disease, according to the Response Evaluation Criteria in Solid Tumors criteria.
From September 2009 to November 2009, the patient received three cycles of topotecan and cisplatin chemotherapy. Bilateral pleural effusion was slightly increased after this treatment. Follow-up pelvis magnetic resonance imaging (MRI) on 24 November 2009 showed recurrence at the right adnexa. In December 2009, a chest-tubing procedure was performed in the right side of the chest due to increased pleural effusion; the amount of drainage was 1,650 mL.
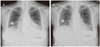
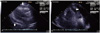
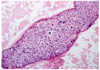
When 5-FU and cisplatin chemotherapy was completed, the patient was treated for general weakness. On 22 December 2009 she suddenly complained of dyspnea and discomfort in the left chest, and symptoms did not subsided with an oxygen mask. Electrocardiogram (ECG) revealed sinus tachycardia and low-voltage QRS waves. Her chest X-ray showed an increase in the size of the cardiac silhouette and small pleural effusion (Fig. 1A). The patient's blood pressure was 90/40 mm Hg, heart rate was 132 and body temperature was 35.5℃. Echocardiogram demonstrated the presence of a large pericardial effusion (Fig. 2). A pericardiocentesis was performed under ultrasound guidance. About 800 mL of fluid was drained, and a catheter was placed and remained for seven days. Cytological examination showed squamous cell carcinoma (Fig. 3). On 28 December 2009, the patient complained of dyspnea and discomfort of the left chest again. ECG showed no pericardial effusion, but the amount of left pleural effusion had increased. A chest-tubing procedure was performed in the left side of the chest, and the amount of drainage was 1,300 mL. After the procedure, the patient was treated with Lasix and Isoket. Sclerosis was performed in the left chest with cisplatin. Her symptoms likely would have improved, but she expired one month after the pericardiocentesis.
Malignant pericardial effusion secondary to pericardial metastasis from squamous cell carcinoma of the uterine cervix represents an infrequently seen, but potentially life-threatening problem. Mean survival periods following diagnosis of a malignant pericardial effusion in patients with solid tumors other than breast cancer are three to four months; in comparison, patients with metastatic breast cancer generally survive for more than nine months [4]. The normally compliant pericardium may slowly collect up to two liters of fluid before tamponade occurs. Early symptoms, which occur in only 5-27% of those affected prior to the hemodynamic consequences of tamponade, include dysphagia or singultus from mass effect, chest pain or pressure, cough, orthopnea, dyspnea, and/or palpitations. Later symptoms of a large effusion include exertional fatigue due to low cardiac output and systemic venous congestion [5]. Physical findings of significant effusion include jugular venous distension, quiet heart sounds, narrow pulse pressure, tachycardia, and pulsus paradoxus greater than 10 mm Hg. ECG changes include atrial arrhythmias, low voltages, atrioventricular block, repolarization changes, and more specifically, electrical alternans [6]. Radiographic studies to detect or determine the nature of effusion include serial chest X-rays showing cardiac enlargement in the absence of pulmonary vascular congestion, especially with a "water-bottle heart" configuration. Management depends on hemodynamic factors. If tamponade is present, immediatede compression by pericardiocentesis or immediate pericardial window in centers so oriented is indicated. Subsequent steps are controversial [7].
Pericardiocentesis is best performed in an intensive-care setting with appropriate monitoring, usually under ultrasound guidance. In this setting, major morbidity is uncommon, but mortalities have been reported from ventricular puncture [8]. Intracavitary instillation of agents such as bleomycin or tetracycline has proven both safe and effective to control malignant pleural effusion, and has experienced with their use in pericardial effusive disease and has confirmed their utility in that setting as well [9].
Tetracycline sclerosis after pericardiocentesis is another conservative therapeutic option. This procedure is relatively simple and is 60-80% effective in controlling symptomatic effusion with few complications in reported series [10]. Prior to 1976, various sources reported that chemotherapy for metastatic cervical squamous cell carcinoma had response rates of 0-25%. Gynecologic Oncology Group experience, reported by Thigpen et al. [11], noted a 38% response to cisplatin 50 mg/m2 each three weeks and responses of 0-19 % for other agents. Selected responses to combined agents were reported by Thigpen et al. [11] to be as high as 93% for mitomycin C and bleomycin, ranging from 6-93% for two-to seven-drug regimens [12].
Repeated aspirations and instillations may be required for satisfactory control of malignant pericalrdial effusion. Adverse effects associated with intrapericardial instillation include severe local pain with mechlorethamine and quinacrine, bone marrow suppression with alkylating agents and rarely sepsis, arrythemias, ventricular laceration, and death [13].
The best candidates for radiotherapy are probably patients with radiosensitive tumors in whom other therapies have failed or who are chemoresistant. Studies examining various primary tumors have reported external radiotherapy success rates ranging from 50-100% for control of pericardial effusion [14].
Surgical management may therefore be reserved for patients with treatment failures, a constrictive component to the physiologic process and/or the need for histologic confirmation. Early detection and prompt management of pericardial effusion in patients with gynecologic malignancies may decrease morbidity and prolong life [5]. Carcinoma of the uterine cervix with extrapelvic metastasis is currently an in curable disease. The occurrence of pericardial effusion in this disease is rare, and yet its early recognition is important to prevent cardiac tamponade [15].
Oncologists have previously regarded the development of a pericardial effusion as a pre-terminal event. More recent data contradict this belief. Aggressive local cardial effusion provides palliation of symptoms and may prolong life in women with cervical cancer [5].
Figures and Tables
Acknowledgments
This study was supported by a research grant from Kosin University College of Medicine, Korea.
References
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008. 58:71–96.
2. Lockwood WB, Broghamer WL Jr. The changing prevalence of secondary cardiac neoplasms as related to cancer therapy. Cancer. 1980. 45:2659–2662.
3. Hayashi Y, Iwasaka T, Hachisuga T, Kishikawa T, Ikeda N, Sugimori H. Malignant pericardial effusion in endometrial adenocarcinoma. Gynecol Oncol. 1988. 29:234–239.
4. Nelson BE, Rose PG. Malignant pericardial effusion from squamous cell cancer of the cervix. J Surg Oncol. 1993. 52:203–206.
5. Rieke JW, Kapp DS. Successful management of malignant pericardial effusion in metastatic squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 1988. 31:338–351.
6. Kralstein J, Frishman WH. Malignant pericardial diseases: diagnosis and treatment. Cardiol Clin. 1987. 5:583–589.
7. Hankins JR, Satterfield JR, Aisner J, Wiernik PH, McLaughlin JS. Pericardial window for malignant pericardial effusion. Ann Thorac Surg. 1980. 30:465–471.
8. Celermajer DS, Boyer MJ, Bailey BP, Tattersall MH. Pericardiocentesis for symptomatic malignant pericardial effusion: a study of 36 patients. Med J Aust. 1991. 154:19–22.
9. Shepherd FA, Morgan C, Evans WK, Ginsberg JF, Watt D, Murphy K. Medical management of malignant pericardial effusion by tetracycline sclerosis. Am J Cardiol. 1987. 60:1161–1166.
10. Shepherd FA, Ginsberg JS, Evans WK, Scott JG, Oleksiuk F. Tetracycline sclerosis in the management of malignant pericardial effusion. J Clin Oncol. 1985. 3:1678–1682.
11. Thigpen T, Vance RB, Balducci L, Blessing J. Chemotherapy in the management of advanced or recurrent cervical and endometrial carcinoma. Cancer. 1981. 48:2 Suppl. 658–665.
12. Choo YC. Chemotherapy in advanced primary and recurrent cervical carcinoma. Int J Gynaecol Obstet. 1982. 20:417–423.
13. Davis S, Rambotti P, Grignani F. Intrapericardial tetracycline sclerosis in the treatment of malignant pericardial effusion: an analysis of thirty-three cases. J Clin Oncol. 1984. 2:631–636.
14. Cham WC, Freiman AH, Carstens PH, Chu FC. Radiation therapy of cardiac and pericardial metastases. Radiology. 1975. 114:701–704.
15. Jamshed A, Khafaga Y, El-Husseiny G, Gray AJ, Manji M. Pericardial metastasis in carcinoma of the uterine cervix. Gynecol Oncol. 1996. 61:451–453.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download