Abstract
Purpose
The purpose of this study was to evaluate the efficacy of narrow-band imaging (NBI) as a diagnostic tool for detecting bladder tumors during cystoscopy compared with white light cystoscopy (WLC).
Materials and Methods
From December 2013 to June 2017, a randomized prospective study was conducted on 198 patients underwent transurethral resection of bladder tumor by a single surgeon. The patients were divided into two groups according to diagnostic method. In Group I, WLC only was performed. In Group II, NBI was additionally performed after WLC. We analyzed the rate of detection of bladder tumors as a primary endpoint. In addition, we evaluated rates of recurrence in each group.
Results
There were no significant differences between the two groups in characteristics except hypertension. In the analysis of rates of detection, the probability of diagnosing cancer was 80.9% (114/141) in the WLC group, and the probability of diagnosing cancer using WLC in the NBI group was 85.5% (159/186). After switching from WLC to NBI for second-look cystoscopy in the NBI group, NBI was shown to detect additional tumors with a detection rate of 35.1% (13/37) from the perspective of the patients and 42.2% (27/64) from the perspective of the tumors. The 1-year recurrence-free rate was 72.2% in the WLC group and 85.2% in the NBI group (p=0.3).
Bladder cancer is among the most common of the urinary tract cancers and is the 11th most common malignancy worldwide [1]. In Korea, bladder cancer was the 2nd most common urologic cancer from 1999 to 2012 [2]. Bladder cancer is divided into two types: non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC); the former accounts for about 75% and the latter about 25% [1]. Currently, the most common method of diagnosing and treating NMIBC is by transurethral resection (TUR) using white light cystoscopy (WLC). However, WLC has several limitations, and there is a high rate of recurrence at the time of NMIBC diagnosis and follow-up. A small, flat lesion that was in fact a tumor or carcinoma in situ (CIS) and that was overlooked at the time of initial diagnosis and surgery can result in recurrence [3]. These undiscovered flat lesions are likely to recur (approximately 61% at year one and 78% at year five) and may even progress to invasive bladder cancer (about 17% at year one and 45% at year five) [45]. Because of these high rates of prevalence and recurrence, bladder cancer is a major burden on medical insurance and the economy [6].
Therefore, a need exists for better detection methods to avoid missing NMIBC. One such method is photodynamic diagnosis (PDD) and another is narrow-band imaging (NBI) cystoscopy. PDD uses porphyrin-induced fluorescence cystoscopy. Photoactive porphyrins, such as hexaminolevulinate or 5-aminolevulinic acid, accumulate preferentially in neoplastic tissue and emit red fluorescence under blue light [3]. NBI uses light at wavelengths of 390 to 445 nm and allows the microvessels to be seen more clearly than with WLC. NBI can detect small lesions or CIS that is not easily seen [7]. NBI is useful in the detection of early-stage cancer in gastrointestinal endoscopy and is therefore expected to play an important role in the detection of NMIBC [8]. In 2008, Bryan et al. [9] published the first study on NBI cystoscopy in 29 patients with recurrent urothelial carcinoma. They concluded that 41% more tumors could be detected with NBI than with WLC. Other studies have also shown that NBI cystoscopy detects more tumors than does WLC [10111213]. In addition, several recent meta-analyses have shown that NBI is effective for detecting flat and abnormal lesions such as CIS. However, despite these reports, NBI is not yet routinely used for diagnosis. This prospective, randomized study compared the rates of detection and recurrence at 1 year between NBI and WLC.
From December 2013 to June 2017, we performed a prospective, randomized, single-blind study to compare WLC and NBI in 198 patients who underwent transurethral resection of a bladder tumor (TURBT) by a single surgeon at a tertiary referral center. The patients were randomly divided into the NBI (experimental) and WLC (control) groups. Randomization was conducted by means of a computer-generated random sequence of numbers (simple randomization). We compared the detection rates between the groups. The study was approved by the institutional review board of our center. This study was registered in the Korea University Anam Hospital clinical trial center (approval number: MD13008).
Patients who underwent TURBT as the result of a suspicion of a bladder tumor discovered by use of cystoscopy or another imaging study were included, whereas patients with muscle-invasive tumors, those undergoing radical cystectomy, those receiving chemotherapy or radiotherapy, and those with nonurothelial carcinoma were excluded. Also, patients who were not histologically diagnosed with cancer, those lost to follow-up, and those who died for other reasons were excluded.
All patients were initially examined with WLC and suspicious lesions were recorded. In the control group, all suspicious areas were resected and histological examination was performed in the operating room. In the experimental group, as in the control group, all suspicious areas identified under WLC were resected and NBI cystoscopy was then performed. Additional resection and biopsy were performed on the suspicious areas under NBI. Both groups were followed for up to 1 year postoperatively, and TUR was performed in patients suspected of having recurrence. Before procedures, patients eligible for the study were contacted by medical staff and provided with verbal and written information. All participants were required to sign informed consent forms.
The primary study endpoint was the identification of tumors in each group and the number of tumors additionally diagnosed as urothelial carcinoma by use of NBI. The secondary endpoint was the recurrence rate after 1 year of follow-up. In addition, outpatient preoperative data including age, sex, weight, height, comorbidity (hypertension, diabetes mellitus, etc.), hematuria, urinalysis, urine culture and cytology, tumor size, and tumor location were analyzed. Postoperative factors that could affect our results were collected and included the duration of surgery, previous TURBT history, and postoperative intravesical therapy (bacille Calmette-Guérin or mitomycin C). Surveillance WLC was planned for all patients at the 1-year follow-up; if tumor recurrence was suspected, histologic confirmation was obtained with TUR.
Student's t-test and the Mann-Whitney test were used to compare variables between the two groups. The estimated recurrence-free rate was obtained by using Kaplan-Meier analysis. The log-rank test was used to compare the Kaplan-Meier curves between the two groups. All analyses were performed by using IBM SPSS Statistics software (ver. 20.0; IBM Co., Armonk, NY, USA). The p-values <0.05 were considered statistically significant.
In addition, we consulted a statistical expert to calculate the appropriate sample size. We determined that 55 patients in each group were needed for a reliability power of 80% with a 10% rate of follow-up loss. This value was obtained by use of the Medcalc (ver. 16.4.1) statistical program. In our study, the actual number of enrolled patients was 198, but 46 patients were excluded by the exclusion criteria. Therefore, the actual number in our study was a total of 152 patients (67 and 85 patients in the WLC and NBI groups, respectively). The flow chart showing patient enrollment is provided in Fig. 1.
From December 2013 to June 2017, a total of 198 patients were enrolled. Of these, 97 were in the WLC group and 101 were in the NBI group. A total of 46 were excluded (MIBC and/or radical cystectomy: 36; systemic chemotherapy: 7; nonurothelial tumor: 3). Of 152 patients who met the criteria, 67 were in the WLC group and 85 were in the NBI group. A flow diagram of the trial protocol is shown in Fig. 1.
The baseline characteristics of the patients are shown in Table 1. Other than hypertension, there were no significant differences between the two groups in age, sex, body mass index, presence of diabetes mellitus, current smoking, smoking duration, or other parameters (Table 1). There was no statistically significant differences in operative time between the two groups (p=0.864), with an average of 30.22 minutes in the WLC group and 29 minutes in the NBI group. In addition, there was no statistically significant difference in hematuria (p=0.472), grade (p=0.93), or cytology (p=0.543) between the two groups. Tumor size (p=0.26) and location (p=0.671) did not differ significantly between the two groups (Table 1).
Twelve patients in the WLC group had no evidence of cancer, 2 had CIS, 37 had Ta cancer, and 16 had T1 cancer (Table 2). Thirteen patients in the NBI group had no evidence of cancer, 3 had CIS, 52 had Ta cancer, and 17 had T1 cancer. The cancer detection rate in the WLC group was 82.1% (55/67), and the probability of diagnosing cancer with first-look WLC in the NBI group was 84.7% (72/85). We then switched from WLC to NBI for second-look cystoscopy. At that time, the number of patients suspected of having a tumor was 37, of whom 24 had no cancer, 6 had CIS, 5 had Ta cancer, and 2 had T1 cancer. The detection rate was 35.1% (13/37). One of these patients had no tumor on WLC but was found to have CIS on NBI cystoscopy.
Of 141 tumors detected in the WLC group, 27 were not cancers; of 114 cases diagnosed with cancer, 11 had CIS, 76 had Ta cancer, and 27 had T1 cancer. Of 186 tumors detected in the NBI group, 27 were not cancers; of 159 cases diagnosed with cancer, 10 had CIS, 108 had Ta cancer, and 41 had T1 cancer on first-look WLC. Therefore, the probability of diagnosing cancer was 80.9% in the WLC group (114/141), and the probability of diagnosing cancer using WLC in the NBI group was 85.5% (159/186). Then, as before, we switched from WLC to NBI for second-look cystoscopy in the NBI group. Of 64 suspicious lesions, 37 were not cancers, 12 were CIS, 11 were Ta cancer, and 4 were T1 cancer. The detection rate was 42.2% (27/64).
Except for those patients with no cancer, a total of 127 remained. At the 1-year follow-up, 74 patients remained after exclusion of those who died for other reasons, those who were lost to follow-up, and those who were excluded for other reasons. Of these 74, 35 were in the WLC group and 39 were in the NBI group.
The recurrence rate in each group is shown in Fig. 2. The 1-year recurrence-free rate was 72.2% in the WLC group and 85.2% in the NBI group, without a statistically significant difference between the groups (p=0.3). However, the recurrence-free rate tended to be greater with NBI than with WLC.
Although approximately 10 years have passed since the introduction of NBI in a urologic setting, it is still not being routinely used in NMIBC. However, several studies have shown that NBI is more effective than WLC in NMIBC. Bryan et al. [9], who first introduced NBI in the urologic setting, found that 15 additional urothelial carcinomas were detected in 12 of 29 patients (41%). These urothelial carcinomas were not found with WLC and could account for early recurrence of disease. In a prospective study published by Cauberg et al. [14] in 2010, TURBT was performed in a total of 95 patients, of whom 78 were diagnosed with urothelial carcinoma. Of these 78, additional tumors were diagnosed in 28 (35.9%) with NBI, and 39 (17.3%) were only found with NBI among a total of 226 tumors. Other studies also concluded that detection rates can be increased with the use of NBI [1516].
In the present study, of 85 patients who underwent NBI cystoscopy, 37 were found to have suspicious areas. Among these, 13 had cancer. In other words, the probability of cancer was 35.2% when additional lesions were discovered with NBI. Thus, the use of WLC alone can miss some cancers. Of the 64 additional tumors detected by NBI, 27 were found to be cancers, for a detection rate of 42.2%. This suggests that NBI can detect more tumors than WLC (Fig. 3).
However, whether this increase in the detection rate results in a decrease in the recurrence rate is unclear. Some previous studies concluded that NBI did not reduce the recurrence rate.
A multicenter, prospective study by Naito et al. [13] published in April 2016 comparing the rate of recurrence at 1 year in NBI and WLC groups showed a significant difference only in low-risk patients. A significantly lower rate of recurrence was found in low-risk patients (pTa, grade 1, <30 mm, and no CIS) in the NBI group than in the WLC group after 3 months (0% vs. 15.1%, p=0.006) and 12 months (5.6% vs. 27.3%, p=0.002) of follow-up. Recurrence rates at 12 months were 27.1% (n=109) and 25.4% (n=104) in the WLC and NBI groups, respectively (p=0.585). The authors suggested that NBI can reduce the recurrence rate by increasing the detection rate in low-risk patients. However, increasing the detection rate in intermediate- or high-risk patients does not significantly decrease the recurrence rate.
Several other studies have shown that recurrence rates can be reduced with NBI. A randomized, prospective trial by Naselli et al. [17] concluded that TUR performed using NBI reduces the recurrence risk of NMIBC. In this study, 1-year recurrence rates were compared in NBI and WLC groups. One-year recurrence was observed in 25 of 76 patients (32.9%) in the NBI group and in 37 of 72 patients in the WLC group (51.4%) (odds ratio, 0.62; p=0.0141). Thus, the rate of recurrence was about 10% lower in the NBI group. Kobatake et al. [18] also reported similar results. The 1-year rate of recurrence was 21.1% (12/57) in the NBI group and 39.7% (31/78) in the WLC group. There was a statistically significant difference (p=0.028).
A systematic review and meta-analysis by Kang et al. [11] in 2017 evaluated six studies to determine whether NBI could reduce the risk of recurrence [1718192021]. NBI-TUR for NMIBC was associated with a significant benefit at 3 months (relative risk [RR], 0.39; 95% confidence interval [CI], 0.26–0.60; p<0.0001), 1 year (RR, 0.52; 95% CI, 0.40–0.67; p<0.00001), and 2 years (RR, 0.60; 95% CI, 0.42–0.85; p=0.004) compared with that for WLC-TUR.
Other than meta-analysis, the most recent studies on NBI in Korea include an article published by Song et al. [22] in 2016. In that study, a total of 63 patients were included. After TURBT by WLC, NBI was used to confirm additional tumors. As a result, NBI showed higher sensitivity (100% vs. 94.1%, p>0.999) and lower specificity (50% vs. 86.9%, p>0.001) than WLC.
In this study, the recurrence-free rate tended to be higher in the NBI group, although the results were not statistically significant. The 1-year rate of recurrence was 27.8% in the WLC group and 14.8% in the NBI group, showing a higher tendency for recurrence in the WLC group. Consequently, an increased rate of detection may seem obvious, but more research is needed to determine whether this leads to an increase in the recurrence-free rate.
A multicenter study published by Drejer et al. [23] in 2017 showed that NBI had higher sensitivity than WLC, and changed the relevant clinical decision in 18 cases (1.9%). In this study, 1 additional cancer (CIS) was detected using NBI in 1 patient (1.18%, 1/85). For this patient, use of NBI found the cancer that was overlooked using only WLC and changed the clinical decision.
NBI is convenient to use. Similar to NBI, PDD provides an enhanced view of bladder cancer. However, PDD requires instillation of a photosensitizing agent into the bladder prior to surgery. NBI, on the other hand, can be performed by pressing a button during WLC. In addition, as shown in this study, there was no significant difference in operative time between NBI and WLC (p=0.864). These methods can be used easily and conveniently during outpatient follow-up.
There were some limitations in this study. First, this was a single-surgeon study. Thus, WLC was followed by second-look NBI performed by the same researcher. When a researcher diagnoses the tissue as cancer through cystoscopy, another researcher can see the same tissue and not diagnose it as cancer. A surgeon's experience and technical abilities may affect all clinical outcomes (including recurrence) after TURBT of a new NMIBC [72425]. A multicenter, multisurgeon, large-scale prospective study may be needed.
Second, an insufficient number of patients were followed for more than 1 year, and no statistically significant difference was observed in survival rates. However, the recurrence rate tended to be lower in the experimental group. As in some other studies, we tried to compare the recurrence rates according to risk, but the number of patients was too small to allow statistical inferences. It is likely that more data will enable meaningful conclusions.
Third, the sensitivity and specificity could not be compared between groups because normal bladder tissue was not collected to prevent injury when determining detection rates. This resulted in higher sensitivity and lower specificity in the NBI group in other studies. Further studies are needed to determine false-negative rates in normal bladder tissue.
NBI was able to detect additional tumors in 35.1% of patients (13/37), and detected 42.2% of tumors (27/64). Although the 1-year recurrence-free rate showed no statistically significant difference between the NBI and WLC groups, the rate tended to be higher in the NBI group. The results of this study have demonstrated the utility of NBI cystoscopy for NMIBC.
Figures and Tables
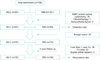 | Fig. 1Flow chart. WLC, white light cystoscopy; NBI, narrow-band imaging; MIBC, muscle-invasive bladder cancer; f/u, follow-up. |
 | Fig. 2Recurrence-free rate in each group. Kaplan-Meier analysis was performed. WLC, white light cystoscopy; NBI, narrow-band imaging. |
 | Fig. 3The figure on the left (A, C, E) is from the white light cystoscopy group and that on the right (B, D, F) is from the narrow-band imaging group. The four top photographs (A~D) are carcinoma in situ and the two bottom photographs (E, F) are Ta. |
Table 1
Patient characteristics of the WLC and NBI groups

Table 2
Detection rate and 1-year recurrence-free rate

References
1. Woldu SL, Bagrodia A, Lotan Y. Guideline of guidelines: nonmuscle-invasive bladder cancer. BJU Int. 2017; 119:371–380.

2. Joung JY, Lim J, Oh CM, Jung KW, Cho H, Kim SH, et al. Current trends in the incidence and survival rate of urological cancers in Korea. Cancer Res Treat. 2017; 49:607–615.

3. Lapini A, Minervini A, Masala A, Schips L, Pycha A, Cindolo L, et al. A comparison of hexaminolevulinate (Hexvix(®)) fluorescence cystoscopy and white-light cystoscopy for detection of bladder cancer: results of the HeRo observational study. Surg Endosc. 2012; 26:3634–3641.

4. Zlatev DV, Altobelli E, Liao JC. Advances in imaging technologies in the evaluation of high-grade bladder cancer. Urol Clin North Am. 2015; 42:147–157.

5. Sylvester RJ, van der, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006; 49:466–475.

6. Sievert KD, Amend B, Nagele U, Schilling D, Bedke J, Horstmann M, et al. Economic aspects of bladder cancer: what are the benefits and costs. World J Urol. 2009; 27:295–300.

7. Bryan RT, Shah ZH, Collins SI, Wallace DM. Narrow-band imaging flexible cystoscopy: a new user's experience. J Endourol. 2010; 24:1339–1343.

8. Hewett DG, Kaltenbach T, Sano Y, Tanaka S, Saunders BP, Ponchon T, et al. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrowband imaging. Gastroenterology. 2012; 143:599–607.

9. Bryan RT, Billingham LJ, Wallace DM. Narrow-band imaging flexible cystoscopy in the detection of recurrent urothelial cancer of the bladder. BJU Int. 2008; 101:702–705.

10. Li K, Lin T, Fan X, Duan Y, Huang J. Diagnosis of narrow-band imaging in non-muscle-invasive bladder cancer: a systematic review and meta-analysis. Int J Urol. 2013; 20:602–609.

11. Kang W, Cui Z, Chen Q, Zhang D, Zhang H, Jin X. Narrow band imaging-assisted transurethral resection reduces the recurrence risk of non-muscle invasive bladder cancer: a systematic review and meta-analysis. Oncotarget. 2017; 8:23880–23890.

12. Xiong Y, Li J, Ma S, Ge J, Zhou L, Li D, et al. A meta-analysis of narrow band imaging for the diagnosis and therapeutic outcome of non-muscle invasive bladder cancer. PLoS One. 2017; 12:e0170819.

13. Naito S, Algaba F, Babjuk M, Bryan RT, Sun YH, Valiquette L, et al. The Clinical Research Office of the Endourological Society (CROES) multicentre randomised trial of narrow band imaging-assisted Transurethral Resection of Bladder Tumour (TURBT) versus conventional white light imaging-assisted TURBT in primary non-muscle-invasive bladder cancer patients: trial protocol and 1-year results. Eur Urol. 2016; 70:506–515.

14. Cauberg EC, Kloen S, Visser M, de la Rosette JJ, Babjuk M, Soukup V, et al. Narrow band imaging cystoscopy improves the detection of non-muscle-invasive bladder cancer. Urology. 2010; 76:658–663.

15. Shen YJ, Zhu YP, Ye DW, Yao XD, Zhang SL, Dai B, et al. Narrow-band imaging flexible cystoscopy in the detection of primary non-muscle invasive bladder cancer: a "second look" matters. Int Urol Nephrol. 2012; 44:451–457.

16. Chen G, Wang B, Li H, Ma X, Shi T, Zhang X. Applying narrow-band imaging in complement with white-light imaging cystoscopy in the detection of urothelial carcinoma of the bladder. Urol Oncol. 2013; 31:475–479.

17. Naselli A, Introini C, Timossi L, Spina B, Fontana V, Pezzi R, et al. A randomized prospective trial to assess the impact of transurethral resection in narrow band imaging modality on non-muscle-invasive bladder cancer recurrence. Eur Urol. 2012; 61:908–913.

18. Kobatake K, Mita K, Ohara S, Kato M. Advantage of transurethral resection with narrow band imaging for non-muscle invasive bladder cancer. Oncol Lett. 2015; 10:1097–1102.

19. Geavlete B, Multescu R, Georgescu D, Stanescu F, Jecu M, Geavlete P. Narrow band imaging cystoscopy and bipolar plasma vaporization for large nonmuscle-invasive bladder tumors--results of a prospective, randomized comparison to the standard approach. Urology. 2012; 79:846–851.

20. Ma T, Wang W, Jiang Z, Shao G, Guo L, Li J, et al. Narrow band imaging-assisted holmium laser resection reduced the recurrence rate of non-muscle invasive bladder cancer: a prospective, randomized controlled study. Zhonghua Yi Xue Za Zhi. 2015; 95:3032–3035.
21. Stănescu F, Geavlete B, Georgescu D, Jecu M, Moldoveanu C, Adou L, et al. NBI - plasma vaporization hybrid approach in bladder cancer endoscopic management. J Med Life. 2014; 7:155–159.
22. Song PH, Cho S, Ko YH. Decision based on narrow band imaging cystoscopy without a referential normal standard rather increases unnecessary biopsy in detection of recurrent bladder urothelial carcinoma early after intravesical instillation. Cancer Res Treat. 2016; 48:273–280.

23. Drejer D, Béji S, Munk Nielsen A, Høyer S, Wrist Lam G, Jensen JB. Clinical relevance of narrow-band imaging in flexible cystoscopy: the DaBlaCa-7 study. Scand J Urol. 2017; 51:120–123.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download