Abstract
Purpose
To verify the quality of pelvic lymph node dissection (PLND) performed at radical prostatectomy (RP) and its impact on nodal recurrence in patients undergoing salvage lymph node dissection (sLND).
Materials and Methods
Retrospective review of 48 patients who underwent sLND for presumed nodal recurrence, to describe the PLND characteristics at RP and correlate the anatomical sites and number of suspicious nodes reported in radiological imaging and final pathology of sLND.
Results
Overall, at RP, 8 (16.7%) did not undergo PLND, 32 (66.7%) and 8 (16.7%) received a “limited” (between external iliac vein and obturator nerve) and an “extended” (external iliac, hypogastric, and obturator) dissection, respectively. Median nodes removed during limited and extended dissection were 2 and 24, respectively. At sLND, the mean age was 61.3 years and median prostate specific antigen (PSA) was 1.07 ng/mL. Median nodes removed at sLND were 17 with a median of 2 positive nodes. Recurrent nodes were identified within the template of an extended PLND in 62.5%, 50.0% and 12.5% patients, respectively, following prior no, limited and extended dissection at RP. Recurrence outside the expected lymphatic drainage pathway was noted in 37.5% patients with prior extended dissection at RP. There was a correlation between imaging and pathology specimen in 83% for node location and 58.3% for number of anatomical sites involved.
In prostate cancer (PCa) patients, pelvic lymph node dissection (PLND) is the most reliable and accurate staging modality for lymph node assessment and is recommended in all patients with intermediate- or high-risk disease undergoing radical prostatectomy (RP) [12]. According to the National Cancer Database, reported rates of PLND in patients undergoing RP for intermediate/high risk PCa was 70.8% with only 26.6% undergoing an extended template (removal of nodal tissue from the external iliac, hypogastric, and obturator areas) PLND [3].
Despite the majority of men undergoing RP for what appears to be clinically localized cancer, up to 40% develop biochemical recurrence (BCR) [45]. Population based analyses have demonstrated that pelvic and/or distant nodal metastasis can represent the only metastatic sites in a portion of patients experiencing BCR [6]. Although nodal recurrences are considered systemic disease and treated with androgen deprivation therapy (ADT), the clinical outcomes of patients presenting with only nodal recurrence appears heterogeneous [7]. Among this cohort of patients presenting with only nodal recurrence, the quality of the PLND performed at RP and its impact on the distribution of nodal recurrence is not known. Since the diagnosis of nodal recurrence is based on the imaging and majority of these patients are treated with medical therapy, pathological confirmation of the topography of the nodal recurrence is often not available.
Salvage lymph node dissection (sLND) can be a treatment option for selected patients with isolated nodal recurrence either as mono or multimodal therapy and recently published midterm oncological outcomes of sLND suggests the possibility of a therapeutic utility [89]. The utilization of sLND has gained increasing popularity over the last decade and sLND provides accurate pathological confirmation of anatomical location and extent of the nodal recurrence. In this study, we examined the patients undergoing sLND for presumed lymph nodal recurrence and analyzed the topography of the pathological confirmed recurrent nodal location and correlated to the quality of PLND performed at the time of RP.
After obtaining Memorial Sloan Kettering Cancer Center Institutional Review Board approval (written informed consent waived) (approval number: 16-1477), 48 patients who underwent sLND between 1998 and 2017 for suspected nodal recurrence after RP were identified and included in the analysis. A prospectively maintained institutional database was queried to obtain the following information-diagnostic prostate biopsy details, description of the operative details of the RP and PLND (type, template, number of nodes removed, node positive rate, final pathology), post-RP follow-up (nadir prostate specific antigen [PSA], BCR, salvage treatments), baseline characters at sLND (age, PSA), pre-sLND imaging (type, node localization, number and the maximal diameter of the identified nodes), sLND details (type, template, number of nodes removed, node positive rate, final pathology), post-sLND follow-up. BCR was defined as PSA >0.1 ng/mL in the post-operative period.
Systemic (non-nodal) recurrent disease was excluded in all patients using conventional imaging such as abdominal computed tomography (CT) scan/magnetic resonance imaging (MRI) and bone scan using technetium Tc 99m methylene diphosphonate. Functional imaging such as positron emission tomogram was used when available. The anatomic location of the nodes in the imaging is reported in the conventional nodal anatomic nomenclature [10]: 1) common iliac lymph nodes: proximity to the common iliac artery and vein, caudal to the aortic bifurcation and cranial to the bifurcation of the common iliac vessels; 2) external iliac lymph nodes: proximity to the external iliac artery and vein, caudal to the bifurcation of the common iliac vessels and cranial to the inguinal ligament; 3) internal iliac/hypogastric lymph nodes: close to the internal iliac vessels (anterior, lateral sacral and presacral); 4) inguinal lymph nodes: located inferior to the level of the inguinal ligament, and inferior to the external iliac node group; 5) aortic nodes: close proximity to the aorta below the inferior mesenteric artery origin (pre and para aortic); 6) caval nodes: close to the inferior vena cava (pre, para, retro and inter-aorto caval); 7) perivisceral: no association with the named blood vessels but in close proximity to visera (perivesical, perirectal). Number of enlarged lymph nodes, lymph node dimensions and avidity on the functional imaging are noted.
The original operative notes were reviewed to identify the template of the PLND performed at RP and at sLND. The standard anatomical landmarks were used to define the boundaries of the templates for LND and numerical values for levels were assigned to specific sets of templates [11].: level 1: external iliac and obturator nodes; level 2: internal iliac/hypogastric nodes; level 3: medial/presacral lymph nodes; level 4: common iliac lymph nodes; level 5: aortic/caval nodes; level 6: inguinal nodes. Since 77% of the primary RP of the included patients was performed at an outside institution, digital copies of the original operative notes were used to assign appropriate levels based on the description of the procedure. All the sLND were performed at our institution and the specimen for pathologic evaluation were named and sent separately based on the nodal packets at the time of operation. Limited PLND was defined as removal of external Iliac and obturator nodes (level 1) and extended PLND was defined as removal of external iliac, obturator and internal iliac nodes (level 1+2). Number of nodes removed, number of positive nodes, anatomical location of the positive nodes, positive node dimensions and the dimension of the tumor focus within the nodes were noted.
We reported means, medians, and interquartile ranges (IQR) for continuous variables. Frequencies and proportions were reported for categorical variables. The analysis performed in the study was to indentify the pattern of nodal recurrence in terms of pelvic nodal anatomic levels based on the template of the PLND at RP. We also correlated the anatomic location and the number of suspicious nodes identified in the pre-sLND imaging with the histologic confirmation of the template based sLND pathology specimen.
Baseline characteristics at RP is shown in Table 1. The mean±standard deviation (SD) age at RP was 56.9±7.7 years and median (IQR) PSA prior to RP was 5.6 (4–9) ng/mL. The Gleason score noted in the pre-RP prostate biopsy was ≥7 (grade group >2) in 41 (85.4%) patients. The primary radical prostatectomies were performed between 1991 and 2015 and was an open or robot assisted procedure in 23 (47.9%) and 25 (52.1%), respectively. Majority of the primary RP (79%) were done in an outside hospital and the decision whether or not to perform a PLND and if performed the extent of PLND was made by the operating surgeon at the time of RP. Based on the operative note and the pathology report, 8 (16.7%) did not undergo PLND at RP, 32 (66.7%) patients had removal of nodal tissue below the external iliac vein and above the obturator nerve (a “limited” dissection), and 8 (16.7%) underwent an “extended” dissection (removal of nodal tissue from the external iliac, hypogastric, and obturator areas) The median (IQR) nodes removed in patient who underwent limited PLND and extended PLND were 2 (2–4) and 24 (18–28), respectively. Five patients (3 limited and 2 extended) had positive node identified at PLND and all had one positive node. Pathologic evaluation of the RP specimen revealed a Gleason score of ≥7 (grade group >2) in 47 (97.9%) and pathological stage was ≥T3 in 38 (79.2%). Extraprostatic extension, seminal vesicle invasion and positive surgical margins were identified in 36 (75.0%), 16 (33.3%) and 21 (43.8%) respectively. Post-RP undetectable PSA was reported in 29 (60.4%) patients and median time to BCR was 24 months. Salvage radiation therapy (RT) and/or ADT for a rising PSA post-RP was given in 32 (66.7%) patients.
sLND were performed in our institution between 1998 and 2017 and the median (IQR) time from RP was 3.5 (2–7.25) years. At sLND, the mean age±SD was 61.3±8.6 years and median (IQR) PSA was 1.07 (0.4–3.2) ng/mL (Table 2). Abdominal CT or MRI was performed in all patients and positron emission tomography (PET) scans were performed in 29 (60.4%) patients with the biologic tracer being fludeoxyglucose, zirconium, choline and prostate-specific membrane antigen (PSMA) in 16, 4, 8 and 1 patient respectively. Pre-sLND imaging identified a single suspicious node in 27 (56.3%) patients and multiple suspicious nodes in 10 (20.8%) patients. Median (IQR) size of the lymph node identified at imaging was 15 (12–18) mm. No suspicious nodes were detected in the imaging in 11 (22.9%) patients (Table 3). However, sLND was performed in the latter group based on several factors such as rising PSA, no other demonstrable focus of disease, prior no/limited PLND during RP, age and pathology of the primary cancer. Pre-sLND biopsy confirmation of the enlarged nodes was done in 7 (14.6%) patients (all were positive for PCa).
sLND was performed as an open, laparoscopic or robot assisted procedure in 3 (6.3%), 25 (52.1%) and 20 (41.7%) patients, respectively. The template of sLND included level 1+2+4 in 46 (95.8%), level 3 in 23 (47.9%), level 5 in 13 (27.1%) and other areas based on imaging in 5 (10.4%) patients. Two patients with isolated PET avid node outside the standard drainage pathway underwent targeted dissection only and no other templates were included in the sLND. Median (IQR) number of nodes removed at sLND was 17 (8–23) with positive nodes being 2 (1–4). The mean (range) largest diameter of the positive node detected at sLND was 19 mm (11–28) mm and the mean (range) largest diameter of the tumor focus noted within the positive nodes was 15 (9–19) mm. sLND was negative for metastatic nodes in 12 (25.0%) patients (Table 4).
Pre-sLND imaging identified 61 suspicious nodes in 52 anatomical locations, but the template mapped sLND histopathologic examination (HPE) of the resected specimen identified 170 metastatic nodes located in 65 anatomic sites. Comparison of the anatomic location of the suspicious nodes detected in the pre-sLND imaging to the final HPE, metastatic nodes were present in the corresponding locations in 83% patients. However, positive nodes were present in additional anatomical sites not identified by imaging. Similarly, imaging underestimated the number of suspicious nodes as compared to positive nodes found in HPE in 28 (58.3%) patients. No metastatic nodes were present in 4 patients with imaging suspicion and 4 patients with negative imaging had positive nodes detected at sLND. Among patients who underwent prior extend PLND at RP the recurrent nodes were located within the anatomical template of level 1 and/or 2 in only 1 (12.5%) patient. Recurrence was noted outside the standard lymphatic drainage pathway of the prostate in 4 (8.3%) patients. Two of these patients had anterior paravesical nodes, one had deep inguinal nodes, and sigmoid mesenteric nodes were positive in one patient. Among the latter group, 3 out of 4 patients had a prior extended PLND at RP. Incidence of finding recurrent nodes in the level 1 and/or 2 template was higher in patients with prior limited or no PLND at RP, 16 (50.0%) and 5 (62.5%) patients respectively.
The median (IQR) follow-up after sLND was 38 (11.5–72) months. A total of 17 (35.4%) patients achieved a non-detectable PSA (<0.05 ng/mL) after sLND. Seven (14.6% of the total group) of these patients continue to have a non-detectable PSA with a median follow-up of 36 (18–37) months; the remaining 10 of the 17 patients achieving a non-detectable PSA subsequently experienced BCR (PSA >0.1 ng/mL). Thirty-one patients (64.6%) had a detectable PSA immediately after sLND.
At the last follow-up, out of 48 patients, 7 (14.6%) were without evidence of disease (PSA <0.1 ng/mL) and not receiving ADT, 25 (52.1%) patients had detectable PSA (>0.1 ng/mL) and without imaging evidence of metastasis (out of which 3 were castrate resistant), 10 (20.8%) patients had imaging evidence of metastasis of which 3 were castrate sensitive and 6 (12.5%) patients died of PCa (Fig. 1).
Anatomical studies suggest that the major pathway for lymph node metastases arising from PCa follow a predictable pattern starting from the periprostatic area; then proceeds in the pelvis through the obturator, internal iliac, external iliac, pararectal, and presacral nodes; further ascends to the common iliac, aortic/caval, retroperitoneal, and mediastinal nodes; eventually draining into the thoracic duct, which in turn opens into the venous system. Few other minor lymphatic pathways exist – anterior route along the paravesical nodes and further along the urachus and more posterior route to perirectal nodes and directly to presacral nodes [10]. Crossover of the lymphatics is well documented with 30%–40% of the lymph node metastases occurring in the contralateral side of the dominant lesions [12]. Predominantly anterior tumors are shown to have significantly lower LN positivity in the conventional PLND template [12].
The utility of PLND at the time of RP is argued, but at minimum a PLND is a diagnostic tool and prognostic indicator after surgery [13]. Men with node positive PCa are at a much higher risk of cancer progression than men who are node negative irrespective of extent of PLND [7]. The optimal extend of PLND for PCa is also often debated. Strong linear association between the number of nodes removed during PLND and node positivity rates has been demonstrated in several studies [14151617181920]. Touijer et al. [21] evaluated 1,269 patients with clinically localized PCa undergoing RP and performed multivariate logistic regression analysis to show the odds of node positivity was 7.5-fold higher while using an extended as compared to a limited PLND. Briganti et al. [18] showed, in a clinical model, that the probability of correctly predicting LN metastasis was close to zero when <10 nodes were removed while a virtually perfect prediction can be attained when ≥30 lymph nodes were removed. Systematic review of published literature on the role of PLND suggested that patients low risk PCa patients may be spared of PLND at RP due low risk of lymph node involvement but when a PLND is indicated during RP, then it should be an extended template [16].
However, impact of performing extended vs limited PLND at RP on the pattern of nodal recurrence is not known. In the present study, we reviewed the final HPE of the template based sLND and compared the anatomic location of the positive nodes to the extent of the primary PLND performed at RP. Only 8 (16.7%) patients in the present cohort had an extended PLND at the time of RP with a median of 24 nodes removed. The pattern of recurrent nodes in this group appears to commonly lie outside the field of initial dissection. This is in marked distinction to men who did not undergo a lymph node dissection at all or only underwent a limited node dissection. In these men, nodal recurrence was noted far more commonly in areas that would have been dissected had an extended template been originally used. While we cannot state de facto that an extended node dissection at the time of RP would have prevented nodal recurrence certainly the implication is to perform the appropriate operation at the original RP.
Another finding noted was pre-sLND imaging appears to underestimate the nodal burden in terms of number and anatomic distribution compared to HPE of resected tissue. While imaging can help to identify suspicious nodes, it does not help define the template of dissection used at sLND. Perhaps with improved imaging techniques, such as PSMA scans, imaging will prove more beneficial in defining the appropriate extent of sLND [22].
The evaluation of the oncological results of this heterogenous group of patients undergoing sLND is beyond the scope of this study but it appears promising. sLND was rarely offered as a monotherapy and most of the patients received ADT as multimodal therapy irrespective of the PSA response. However, we noted that 17 (35.4%) patients achieved a non-detectable PSA (<0.05 ng/mL) after sLND and overall 7 (14.6%) continue to have a non-detectable PSA with a median follow-up of 36 months. Ploussard et al. [23] performed a systematic review of management of node only recurrence after primary local treatment for PCa. Most patients who underwent RP and had a BCR received initial salvage RT followed by ADT. ADT appears to improve PSA-progression free survival but its impact on specific end points like cancer specific and overall survival is unclear. Also there were no studies specifically addressing the node only recurrence in PCa. The experience of salvage RT for node recurrence is very limited a short follow-up. The response rate papers to vary between 13%–75% while 50%–60% of these patients also had concurrent ADT. The authors of the systematic review conclude that highest level of evidence in the management of node only recurrence in PCa is missing and salvage treatments directly addressing the nodal recurrence can have good oncologic outcomes and may postpone systemic treatment [23].
Our study has several limitations–The retrospective nature of the study inherently predisposes to error while interpreting the operative notes. Not all the primary RP specimens were reviewed by our institutional pathologists and hence this could have contributed to non-uniformity of the reporting. Though all the patients were evaluated with a CT/MRI, functional imaging was used (58.3%) only when available. At sLND, level 1+2+4 dissection was performed in 96% of patients but the proximal extension of the template was variable and was dependent on the presence of imaging suspicion of proximal nodes and our evolving concept of sLND over the time. The imaging modalities and extend of dissection were heterogenous in the study cohort since the patients were treated across a long period of time (1998–2007) and there were significant improvement in imaging technology and the selection of patients for salvage PLND has evolved over the timeframe of this study. Some in our series had no imaging evidence of adenopathy prior to salvage PLND. These cases were early in our experience. With improved imaging we now reserve salvage PLND for those with imaging evidence of disease. Finally, the number of patients in our cohort was too small to confirm the significance of the differences in the pattern noted and derive any clinical implications. Despite these limitations, our cohort of patients represents a unique population with pathology confirmed nodal recurrence.
In the majority of PCa patients undergoing sLND, the quality of lymphadenectomy during RP was inadequate. The incidence of nodal recurrence in the anatomical template of external iliac, internal iliac and obturator regions appears to be lower in patients with prior extended pelvic lymphadenectomy at RP. There was also higher incidence of nodal recurrence outside the standard lymphatic drainage pathway in this group of patients. The imaging strategies to detect nodal recurrence appear to underestimate the overall burden of the recurrent disease.
References
1. Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017; 71:618–629. PMID: 27568654.

2. American Urological Association. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline [Internet]. Linthicum: American Urological Association;2017. cited 2017 Apr 5. Available from: https://www.auanet.org/education/guidelines/prostate-cancer.cfm.
3. Wang EH, Yu JB, Gross CP, Smaldone MC, Shah ND, Trinh QD, et al. Variation in pelvic lymph node dissection among patients undergoing radical prostatectomy by hospital characteristics and surgical approach: results from the National Cancer Database. J Urol. 2015; 193:820–825. PMID: 25242393.

4. Suardi N, Porter CR, Reuther AM, Walz J, Kodama K, Gibbons RP, et al. A nomogram predicting long-term biochemical recurrence after radical prostatectomy. Cancer. 2008; 112:1254–1263. PMID: 18286530.

5. Boorjian SA, Thompson RH, Tollefson MK, Rangel LJ, Bergstralh EJ, Blute ML, et al. Long-term risk of clinical progression after biochemical recurrence following radical prostatectomy: the impact of time from surgery to recurrence. Eur Urol. 2011; 59:893–899. PMID: 21388736.

6. Gandaglia G, Abdollah F, Schiffmann J, Trudeau V, Shariat SF, Kim SP, et al. Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate. 2014; 74:210–216. PMID: 24132735.

7. Yossepowitch O, Bianco FJ Jr, Eggener SE, Eastham JA, Scher HI, Scardino PT. The natural history of noncastrate metastatic prostate cancer after radical prostatectomy. Eur Urol. 2007; 51:940–947. discussion 947-8. PMID: 17125912.

8. Suardi N, Gandaglia G, Gallina A, Di Trapani E, Scattoni V, Vizziello D, et al. Long-term outcomes of salvage lymph node dissection for clinically recurrent prostate cancer: results of a single-institution series with a minimum follow-up of 5 years. Eur Urol. 2015; 67:299–309. PMID: 24571959.

9. Zattoni F, Nehra A, Murphy CR, Rangel L, Mynderse L, Lowe V, et al. Mid-term outcomes following salvage lymph node dissection for prostate cancer nodal recurrence status post-radical prostatectomy. Eur Urol Focus. 2016; 2:522–531. PMID: 28723518.

10. Paño B, Sebastià C, Buñesch L, Mestres J, Salvador R, Macías NG, et al. Pathways of lymphatic spread in male urogenital pelvic malignancies. Radiographics. 2011; 31:135–160. PMID: 21257939.

11. Mattei A, Fuechsel FG, Bhatta Dhar N, Warncke SH, Thalmann GN, Krause T, et al. The template of the primary lymphatic landing sites of the prostate should be revisited: results of a multimodality mapping study. Eur Urol. 2008; 53:118–125. PMID: 17709171.

12. Tokuda Y, Carlino LJ, Gopalan A, Tickoo SK, Kaag MG, Guillonneau B, et al. Prostate cancer topography and patterns of lymph node metastasis. Am J Surg Pathol. 2010; 34:1862–1867. PMID: 21107093.

13. Fossati N, Willemse PM, Van den Broeck T, van den Bergh RCN, Yuan CY, Briers E, et al. The benefits and harms of different extents of lymph node dissection during radical prostatectomy for prostate cancer: a systematic review. Eur Urol. 2017; 72:84–109. PMID: 28126351.

14. Allaf ME, Palapattu GS, Trock BJ, Carter HB, Walsh PC. Anatomical extent of lymph node dissection: impact on men with clinically localized prostate cancer. J Urol. 2004; 172:1840–1844. PMID: 15540734.

15. Bader P, Burkhard FC, Markwalder R, Studer UE. Is a limited lymph node dissection an adequate staging procedure for prostate cancer? J Urol. 2002; 168:514–518. discussion 518. PMID: 12131300.

16. Briganti A, Blute ML, Eastham JH, Graefen M, Heidenreich A, Karnes JR, et al. Pelvic lymph node dissection in prostate cancer. Eur Urol. 2009; 55:1251–1265. PMID: 19297079.

17. Briganti A, Chun FK, Salonia A, Gallina A, Farina E, Da Pozzo LF, et al. Validation of a nomogram predicting the probability of lymph node invasion based on the extent of pelvic lymphadenectomy in patients with clinically localized prostate cancer. BJU Int. 2006; 98:788–793. PMID: 16796698.

18. Briganti A, Chun FK, Salonia A, Gallina A, Zanni G, Scattoni V, et al. Critical assessment of ideal nodal yield at pelvic lymphadenectomy to accurately diagnose prostate cancer nodal metastasis in patients undergoing radical retropubic prostatectomy. Urology. 2007; 69:147–151. PMID: 17270638.

19. Heidenreich A, Varga Z, Von Knobloch R. Extended pelvic lymphadenectomy in patients undergoing radical prostatectomy: high incidence of lymph node metastasis. J Urol. 2002; 167:1681–1686. PMID: 11912387.

20. Masterson TA, Bianco FJ Jr, Vickers AJ, DiBlasio CJ, Fearn PA, Rabbani F, et al. The association between total and positive lymph node counts, and disease progression in clinically localized prostate cancer. J Urol. 2006; 175:1320–1324. discussion 1324-5. PMID: 16515989.

21. Touijer K, Rabbani F, Otero JR, Secin FP, Eastham JA, Scardino PT, et al. Standard versus limited pelvic lymph node dissection for prostate cancer in patients with a predicted probability of nodal metastasis greater than 1%. J Urol. 2007; 178:120–124. PMID: 17499306.

22. Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, et al. Sensitivity, specificity, and predictors of positive (68)Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016; 70:926–937. PMID: 27363387.
23. Ploussard G, Almeras C, Briganti A, Giannarini G, Hennequin C, Ost P, et al. Management of node only recurrence after primary local treatment for prostate cancer: a systematic review of the literature. J Urol. 2015; 194:983–988. PMID: 25963190.

Fig. 1
Kaplan-Mein cancer specific survival probability of patients undergoing salvage lymph node dissection.
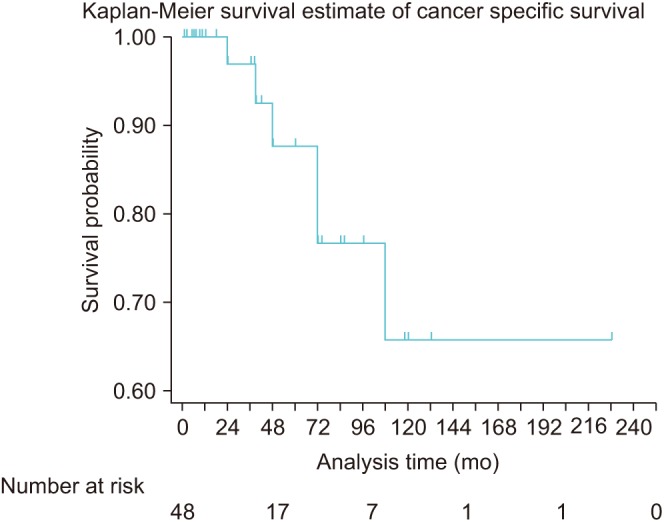
Table 1
Baseline characteristics at RP/PLND (n=48)
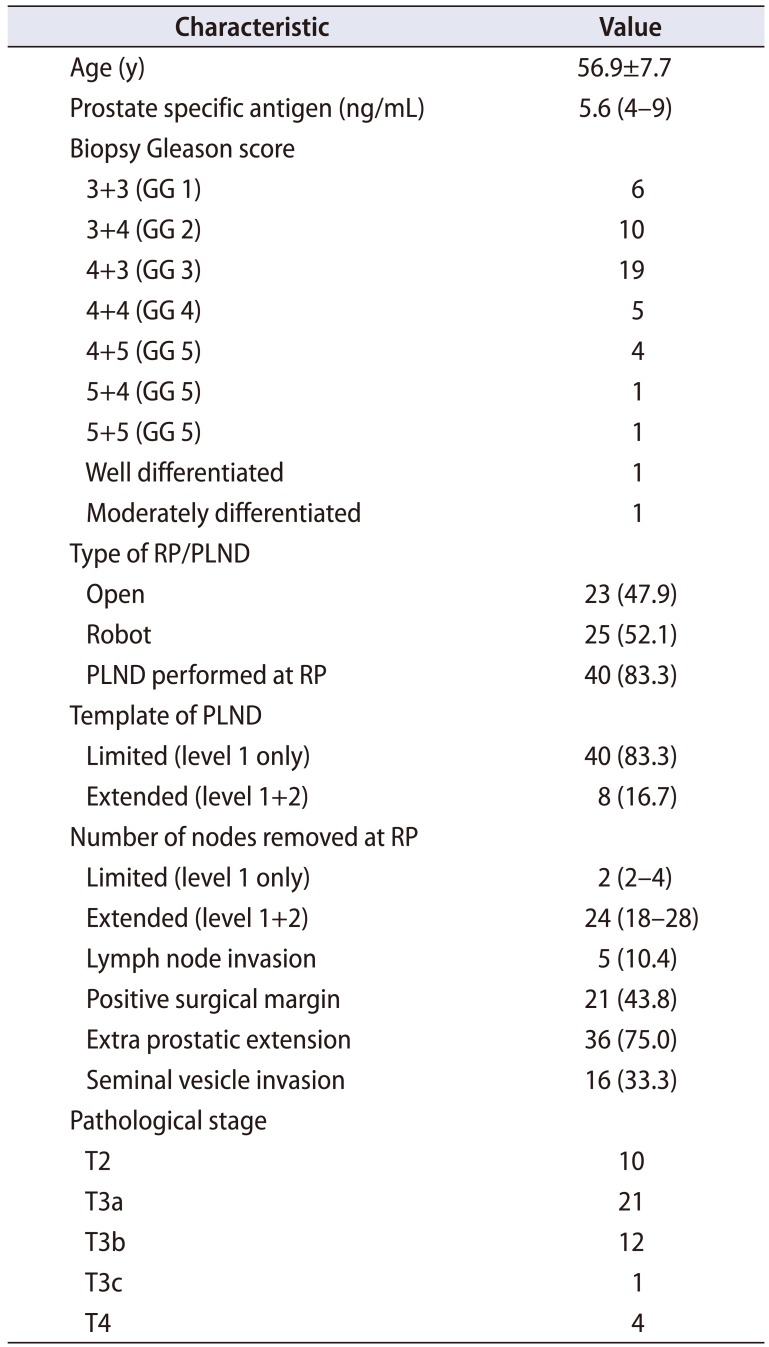
Table 2
sLND characteristics (n=48)
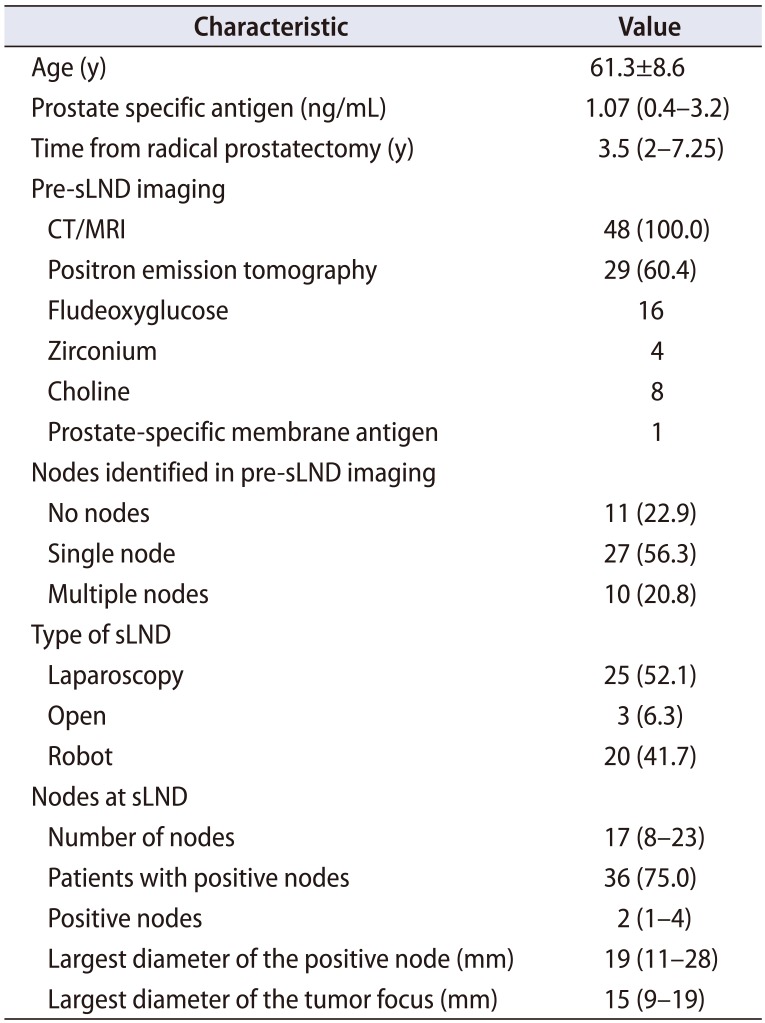
Table 3
List of patients underwent sLND with negative imaging
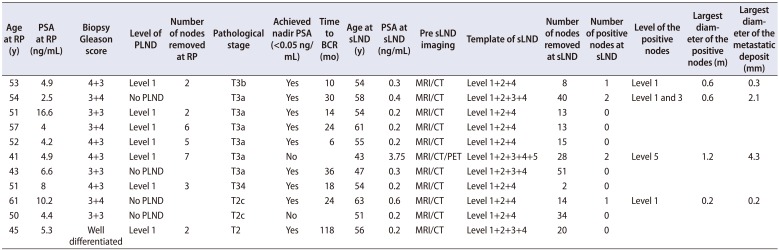
Table 4
List of patients negative for metastatic nodes at sLND




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download