Abstract
Purpose
To evaluate the impact of various computed tomography scan-based parameters of renal stones on the outcome of extracorporeal shock wave lithotripsy (ESWL).
Materials and Methods
We conducted a retrospective study of patients who underwent ESWL for renal stones (sized 5–20 mm) from January 2013 to December 2016. We evaluated body mass index, location of the stone, skin-to-stone distance (SSD), stone attenuation value (SAV), stone diameter, Hounsfield density, stone area, and stone volume. Statistical analysis was done and significance was confirmed by multivariate logistic regression analysis.
Results
Of the 203 patients 122 (60.1%) had successful clearance of the stone. The presence of a double J stenting, a lower pole location, a higher SAV, higher Hounsfield density, larger stone area, larger stone diameter, and higher stone volume were negative predictors of ESWL outcome. When these parameters were analyzed with multivariate logistic regression analysis, stone location, SSD, and SAV were the only significant independent predictors of the outcome of ESWL.
Urinary stones, or urolithiasis, are one of the most common pathologies in human medical science [12]. Urinary stones recur in two-thirds of patients within 20 years and are estimated to affect 12% of men and 5% of women in their lifetime [1]. The incidence rate of urinary stones has been noted to be increasing in both developed and developing countries over the past few decades. This increase may be attributed to modern lifestyles, in particular the increase in obesity [3].
As Pakistan is located in the “stone belt” region, the incidence of urinary stones is high [24]. Stone disease accounts for more than half of all patients in the outpatient department and more than one-third of all urological admissions in tertiary health care centers in Pakistan [4].
The disease usually manifests in the fourth and fifth decades of life. Stone location in the urinary tract can vary. In developed countries, 97% of stones are located in the kidney and ureter. Various management options exist for urolithiasis, including a conservative approach, surgical approaches, and extracorporeal shock wave lithotripsy (ESWL), with the decision based on evaluation by a professional medical team [1].
ESWL, after its introduction in 1980, revolutionized the treatment of urinary stones owing to its noninvasive and ambulatory nature along with the lack of requirement for anesthesia [5]. ESWL is considered as the first-line treatment of urinary tract stones and the success rate is reported to be 80%–90% [16].
Imaging has an important role in urolithiasis and aids not only in the initial diagnosis but also in planning treatment and follow-up of patients with renal and ureteric stones. Since the 1990s, noncontrast computed tomography (NCCT) has become the gold standard imaging modality. Its advantages are its high sensitivity and specificity (>95% and >96%, respectively), availability, faster speed of acquisition, avoidance of intravenous contrast, and the ability to exclude other conditions that may mimic renal colic [3]. NCCT not only provides information regarding urinary tract abnormalities but is also helpful in determining the stone location, size, shape, density, and skin-to-surface distance [6].
Multiple studies have evaluated imaging-based parameters and their effects on the success of ESWL with varying results. In our study, we attempted to investigate various radiological parameters determined by NCCT scan and their ability to predict the outcome of ESWL for renal stones.
All patients diagnosed with urinary stones between January 2013 and December 2016 who had undergone ESWL were considered in the study. The exclusion criteria were patients who had 1) ureteric stones, 2) stone size <5 mm or >20 mm, 3) multiple renal stones, 4) structural urinary tract abnormalities, 5) an absolute contraindication to ESWL such as pregnancy or coagulopathy, 6) undergone a surgical procedure such as percutaneous nephrolithotomy or endoscopic treatment before ESWL, 7) a solitary kidney, and 8) not undergone an NCCT scan before the procedure or had incomplete follow-up. The study was approved by the Institutional Review Board and Ethical Committee of Shifa International Hospital (approval number: 850-125-2017).
Prior to ESWL, each patient was assessed by a medical history, physical examination, urinalysis, urine culture, serum chemistry profile, coagulation profile, and NCCT. None of the patients was prescribed prophylactic antibiotics or analgesia before the procedure. ESWL was performed by a dedicated physician. The number of shock waves and energy settings were decided by the physician. A maximum of 4,000 shock waves were administered at a maximum power of 18 kV. During ESWL, pain was managed with intravenous nalbuphine. The stones were fragmented under fluoroscopic or ultrasound guidance.
During the procedure, the patient's vital signs, including pulse, blood pressure, and oxygen saturation, were monitored. After the procedure, patients were prescribed diclofenac sodium and tamsulosin to take at home. Patients were followed up 2 weeks later by kidney, ureter, bladder (KUB) X-ray, ultrasonography, or both. Treatment was repeated if there was no or inadequate (stone fragment >4 mm) fragmentation. Patients in whom no or poor stone fragmentation was noted 40 days after the procedure were considered failures.
We used a multi-detector CT scanner (3.0 mm/120 kV/200 mAs, Aquilion One, Merge Health Care 2006 & 2010, Chicago, IL, USA) for imaging and a Modulith SLX lithotripter (4th generation; STORZ Medical Equipment, Tägerwilen, Switzerland) for ESWL. All radiological parameters were measured by a postgraduate resident. Maximum stone diameter was measured in the image as the stone diameter that yielded the highest value. Stone attenuation value (SAV) was measured by creating three regions of interest in three different views of the stone on the CT scan showing the stone in the largest dimension. The average of three regions of interest represented the mean Hounsfield unit (HU) for that stone. Care was taken to not include soft tissue. Skinto-stone distance (SSD) was measured as an average of the distance between skin and stone at 0°, 450°, and 900° on NCCT. Stone area was measured by tracing the contour of the inner edge of the stone (not including the surrounding soft tissue) on the slice with maximum stone cross-sectional area. This illustration was then used to automatically calculate stone area. Stone volume was calculated by the equation: l. w. d. π. 0.167 (π=3.14159), where l is length, w is width, and d is depth [7]. Hounsfield density was measured by dividing the HU by stone diameter. The outcome was considered a success when there was no residual stone or residual stone fragments less than 40 mm after 40 days of follow-up.
Patients were then divided into multiple groups based on various parameters and the success rates of ESWL were compared. Patients with stone diameter less than 10 mm and stone diameter greater than 10 mm were divided into separate groups. Then, three groups were made on the basis of SAV <500 HU, 500 to 1,000 HU, and >500 HU. Another parameter was SSD with one group having SSD ≤100 mm and the second group having SSD >100 mm. The last parameter on the basis of which the patients were divided into groups was stone volume: volume ≤500 mm3 in one group and >500 mm3 in the other group.
All statistical analyses were performed by using the student's t-test, Pearson's chi-square test, Mann Whitney U test, and multivariate logistic regression analysis. Normality of numerical data was tested with the Kolmogorov-Smirnov test. Therefore, nonparametric analysis was used to determine the difference and association. Logistic regression was used to calculate the crude odds ratio of all potential independent factors on the outcome. Multivariate logistic regression, with backward stepwise procedures, was used to control for confounding variables and to calculate adjusted odds ratios. SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA) was used, and p-values less than 0.05 were considered statistically significant.
Urinary stones were diagnosed in 3,060 patients, who underwent ESWL treatment in the urology department at Shifa International Hospital Islamabad. Among them, 1,346 patients had a NCCT scan done before the procedure. The total number of patients fulfilling the study criteria was 203. Table 1 describes the characteristics of the patient population.
Of the total patients, 122 patients (60.1%) experienced successful clearance of the stone. The patients consisted of 141 men (69.5%) and 62 women (30.5%). The patients' mean age was 41.49±14.30 years. Forty-one patients (20.2%) patients had double J (DJ) stents in place when ESWL was performed. The success rate was 39.0% (16/41) in those who had DJ stents in place, whereas it was 65.4% (106/162) in those who did not have DJ stents (p=0.002). Stone clearance was 84.0%, 70.3%, and 53.2% for radiolucent, faintly radioopaque, and radio-opaque stones, respectively (p=0.006). Lower pole stones had a lower stone clearance (51.4%) compared with stones located in the pelvis, middle pole, and upper pole (73.1%, 73.3%, and 59.3% respectively; p=0.038).
As shown in Table 2, the median stone diameter in the success and failure groups was 9.39 mm and 13.41 mm, respectively (p<0.001). The median SAV of stones in the success and failure groups was 601.65 HU and 999.0 HU, respectively (p=0.001), whereas the Hounsfield densities were 67.97 HU/mm and 73.77 HU/mm, respectively (p=0.008). Median stone area and volume were significantly lower in the success group (46.59 mm2 and 323.63 mm3, respectively) than in the failure group (81.81 mm2 and 657.8 mm3, respectively; p<0.001 in each group).
Analysis was then performed according to groups based on the parameters mentioned earlier. The success rate in patients with a stone diameter <10 mm was 79.3% but decreased to 45.7% in those with stone diameter >10 mm (p<0.001). Regarding SAV, success rates were 93.8%, 62.7%, and 24.5% in the groups with SAV <500 HU, 500 to 1,000 HU, and >1,000 HU, respectively (p<0.001). Those patients with SSD ≤100 mm had a success rate of 71.4%, but this decreased to 46.2% in those with SSD >100 mm (p<0.001). In reference to stone volume, the success rate was 70.6% in the group with stone volume ≤500 mm3 compared with 45.2% in the group with stone volume >500 mm3 (p<0.001).
When multivariate analysis with logistic regression was performed, SAV was the strongest predictive factor (odds ratio, 1.004 [1.002–1.006]; p<0.001). SSD and stone location were other parameters that showed correlation. Table 3 describes the results of the multivariate analysis, and Table 4 details the correlation of these parameters with the outcome of ESWL.
Much ef fort is being put into investigating the various parameters that may aid in the decision for the management of urinary stones [8]. We analyzed some of these parameters with regard to their impact on renal stones in patients undergoing ESWL.
In 1994, Lingeman et al. [9] demonstrated that clearance of stones in the lower pole calyx by ESWL was lower than the clearance of stones in other calyces. Further studies emphasized this finding, with one study showing a success rate of 69% for lower pole stones compared with 90%, 87%, 85%, and 84% for stones in the renal pelvis, middle calyx, upper ureter, and upper calyx, respectively [10], and another study showing a 25% success rate for stones in the lower pole compared with 40% for stones in the other poles [11]. This difference may be attributable to the narrow infundibulopelvic angle resulting in the incomplete clearance of stone fragments [12].
These studies were followed by efforts by Mostafavi et al. [13] in 1998, Motley et al. [14] in 2001, Patel et al. [15] in 2009, and Spettel et al. [16] in 2013 to use SAVs, in HU, as a reliable parameter for determining stone composition and density that would in turn affect the success rate of ESWL. In 2005, Gupta et al. [17] and Pareek et al. [8] concluded that with an increase in stone density, the requirement for shock wave energy increases. Furthermore, in 2013, Hameed et al. [18] reported similar results and concluded that stones having HU >1,350 require increased shock wave energy. Other studies have contested this value, although with a similar conclusion that the increase in stone density is associated with an increase in shock wave energy requirement [192021].
In 2014, Massoud et al. [10] furthered this effort by categorizing patients with upper urinary tract stones into three groups, <500 HU, 500 to 1,000 HU, and >1,000 HU, with success rates of 100%, 95.7%, and 44.6%, respectively, while defining the cutoff value of 956.5 HU. Some studies even suggested the use of HU density as a better indicator than the HU value alone, especially with regard to differences in radiodensities among urinary stones [14]. Similarly, stone size, stone area, and stone volume have also been reported in various studies to affect the outcome, with decreases in these values being directly associated with improved success rates [222324].
In 2012, Park et al. [6] described SSD as the only significant factor predicting the ESWL success rate and reasoned that with increases in the SSD, shock waves would have to travel a greater distance and penetrate through excess body fat, causing energy levels to decrease and resulting in lower stone fragmentation capability. A similar correlation was reported in multiple other studies [51225]. Badran et al. [26] went on to suggest that shock waves lose their energy by 10% to 20% for every 6-cm penetration. Perks et al. [27] defined 90 mm as the threshold value for SSD beyond which the success rate decreases.
Our study confirmed the validity of the parameters suggested in these previous studies. Stone clearance for lower pole stones was only 51.4% compared with 73.1% for renal pelvis stones and 73.3% for midpole stones. Patients having SAVs less than 500 HU were much more likely to have stone clearance (93.8%), while those having values greater than 1,000 HU were much less likely to experience successful outcomes (24.5%), even with an increasing number of shock waves. Multivariate analysis did prove a correlation between the SAV and success rate, with an odds ratio of 1.004 (95% confidence interval [CI], 1.002 to 1.006; p<0.001).
While there was a difference in the success rate when HU density was analyzed, multivariate analysis failed to show any significance in the difference. Similarly, correlation was also noted between the stone burden (in terms of stone size or diameter, stone area, and stone volume) and the success rate. However, this again failed to show any significance in the multivariate analysis. This may be attributable to the homogeneity of stone size within our study population resulting in a type I error.
For analyzing the correlation of the success rate with SSD, we defined 100 mm as the cutoff value as used by Park et al. [6]. A success rate of 71.4% was noted in patients having SSD less than 100 mm in contrast to 46.2% in those with SSD greater than 100 mm. Multivariate analysis confirmed this significance, resulting in an odds ratio of 1.036 (95% CI, 1.014 to 1.059; p=0.001). Moreover, SSD was noted to be 90.65 mm in the success group compared with 104.33 mm in the failure group, which favors the cutoff value suggested by Perks et al. [27].
The retrospective nature of our study was a disadvantage that we tried to overcome by multivariate analysis as best we could. Similarly, many of the patients did not have an NCCT scan done in the follow-up period. However, ultrasonography or KUB X-ray was done, which might have been reliable enough considering that no recurrence in symptoms or abnormal findings were detected in the imaging.
We also did not consider factors not relating to the NCCT scan that might affect the success rate of ESWL, such as complications. Pain, a complication of the procedure, has been shown to affect the success rate for ESWL [28], as have other complications that may reduce patient compliance, resulting in difficulty targeting the stones.
Better patient selection is paramount to improving the outcome of ESWL. The stone parameters measured by NCCT scan are simple, readily available, reproducible, and predictive. Renal stone location, SAV, and SSD were shown to be independent predictors of ESWL outcome and useful tools for planning the treatment of renal stones. These parameters should be considered when making decisions regarding the use of ESWL.
Figures and Tables
Table 1
General characteristics of the patients
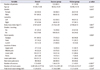
Table 2
Effect of different variables on the outcome of extracorporeal shock wave lithotripsy
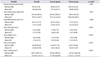
Table 3
Results of the multivariate logistic regression analysis

Table 4
Correlation of variable groups with the outcome of extracorporeal shock wave lithotripsy

References
1. Junuzovic D, Prstojevic JK, Hasanbegovic M, Lepara Z. Evaluation of Extracorporeal Shock Wave Lithotripsy (ESWL): efficacy in treatment of urinary system stones. Acta Inform Med. 2014; 22:309–314.

2. Jan H, Akbar I, Kamran H, Khan J. Frequency of renal stone disease in patients with urinary tract infection. J Ayub Med Coll Abbottabad. 2008; 20:60–62.
3. Andrabi Y, Patino M, Das CJ, Eisner B, Sahani DV, Kambadakone A. Advances in CT imaging for urolithiasis. Indian J Urol. 2015; 31:185–193.

4. Buchholz NP, Abbas F, Afzal M, Khan R, Rizvi I, Talati J. The prevalence of silent kidney stones--an ultrasonographic screening study. J Pak Med Assoc. 2003; 53:24–25.
5. Ather MH, Abid F, Akhtar S, Khawaja K. Stone clearance in lower pole nephrolithiasis after extra corporeal shock wave lithotripsy-the controversy continues. BMC Urol. 2003; 3:1.

6. Park BH, Choi H, Kim JB, Chang YS. Analyzing the effect of distance from skin to stone by computed tomography scan on the extracorporeal shock wave lithotripsy stone-free rate of renal stones. Korean J Urol. 2012; 53:40–43.

7. Al-Ali BM, Patzak J, Lutfi A, Pummer K, Augustin H. Impact of urinary stone volume on computed tomography stone attenuations measured in Hounsfield units in a large group of Austrian patients with urolithiasis. Cent European J Urol. 2014; 67:289–295.

8. Pareek G, Armenakas NA, Panagopoulos G, Bruno JJ, Fracchia JA. Extracorporeal shock wave lithotripsy success based on body mass index and Hounsfield units. Urology. 2005; 65:33–36.

9. Lingeman JE, Siegel YI, Steele B, Nyhuis AW, Woods JR. Management of lower pole nephrolithiasis: a critical analysis. J Urol. 1994; 151:663–667.

10. Massoud AM, Abdelbary AM, Al-Dessoukey AA, Moussa AS, Zayed AS, Mahmmoud O. The success of extracorporeal shock-wave lithotripsy based on the stone-attenuation value from non-contrast computer tomography. Arab J Urol. 2014; 12:155–161.
11. Geraghty R, Burr J, Simmonds N, Somani BK. Shock wave lithotripsy outcomes for lower pole and non-lower pole stones from a university teaching hospital: parallel group comparison during the same time period. Urol Ann. 2015; 7:46–48.

12. Semins MJ, Matlaga BR. Strategies to optimize shock wave lithotripsy outcome: patient selection and treatment parameters. World J Nephrol. 2015; 4:230–234.

13. Mostafavi MR, Ernst RD, Saltzman B. Accurate determination of chemical composition of urinary calculi by spiral computerized tomography. J Urol. 1998; 159:673–675.

14. Motley G, Dalrymple N, Keesling C, Fischer J, Harmon W. Hounsfield unit density in the determination of urinary stone composition. Urology. 2001; 58:170–173.

15. Patel SR, Haleblian G, Zabbo A, Pareek G. Hounsfield units on computed tomography predict calcium stone subtype composition. Urol Int. 2009; 83:175–180.

16. Spettel S, Shah P, Sekhar K, Herr A, White MD. Using Hounsfield unit measurement and urine parameters to predict uric acid stones. Urology. 2013; 82:22–26.

17. Gupta NP, Ansari MS, Kesarvani P, Kapoor A, Mukhopadhyay S. Role of computed tomography with no contrast medium enhancement in predicting the outcome of extracorporeal shock wave lithotripsy for urinary calculi. BJU Int. 2005; 95:1285–1288.

18. Hameed DA, Elgammal MA, ElGanainy EO, Hageb A, Mohammed K, El-Taher AM, et al. Comparing non contrast computerized tomography criteria versus dual X-ray absorptiometry as predictors of radio-opaque upper urinary tract stone fragmentation after electromagnetic shockwave lithotripsy. Urolithiasis. 2013; 41:511–515.

19. El-Assmy A, El-Nahas AR, Abou-El-Ghar ME, Awad BA, Sheir KZ. Kidney stone size and hounsfield units predict successful shockwave lithotripsy in children. Urology. 2013; 81:880–884.

20. Ouzaid I, Al-qahtani S, Dominique S, Hupertan V, Fernandez P, Hermieu JF, et al. A 970 Hounsfield units (HU) threshold of kidney stone density on non-contrast computed tomography (NCCT) improves patients' selection for extracorporeal shock-wave lithotripsy (ESWL): evidence from a prospective study. BJU Int. 2012; 110:E438–E442.

21. Foda K, Abdeldaeim H, Youssif M, Assem A. Calculating the number of shock waves, expulsion time, and optimum stone parameters based on noncontrast computerized tomography characteristics. Urology. 2013; 82:1026–1031.

22. McClain PD, Lange JN, Assimos DG. Optimizing shock wave lithotripsy: a comprehensive review. Rev Urol. 2013; 15:49–60.
23. Tanaka M, Yokota E, Toyonaga Y, Shimizu F, Ishii Y, Fujime M, et al. Stone attenuation value and cross-sectional area on computed tomography predict the success of shock wave lithotripsy. Korean J Urol. 2013; 54:454–459.

24. Bandi G, Meiners RJ, Pickhardt PJ, Nakada SY. Stone measurement by volumetric three-dimensional computed tomography for predicting the outcome after extracorporeal shock wave lithotripsy. BJU Int. 2009; 103:524–528.

25. Pareek G, Hedican SP, Lee FT Jr, Nakada SY. Shock wave lithotripsy success determined by skin-to-stone distance on computed tomography. Urology. 2005; 66:941–944.

26. Badran YA, Abdelaziz AS, Shehab MA, Mohamed HA, Emara AA, Elnabtity AM, et al. Is scoring system of computed tomography based metric parameters can accurately predicts shock wave lithotripsy stone-free rates and aid in the development of treatment strategies? Urol Ann. 2016; 8:197–202.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download