Abstract
Purpose
To report our initial experience with urethral reconstruction using a combined dorsal lingual mucosal graft (LMG) and ventral onlay preputial flap for long obliterative or near-obliterative strictures in circumcised patients.
Materials and Methods
This was a retrospective study of 10 patients from January 2015 to June 2017 with long obliterative or near-obliterative anterior urethral strictures and circumcised prepuces. All patients underwent a combined approach using a dorsally LMG and a narrow preputial onlay flap ventrally to create a 26–30 Fr. neourethra over a 14-Fr Foley catheter. Success was defined as no requirement for additional urethral instrumentation. The follow-up period ranged from 6 to 32 months.
Results
The patients ranged in age from 17 to 44 years (mean, 32.3±9.59 years) and stricture length ranged from 9 to 12.5 cm (mean, 10.77±1.15 cm). Four strictures were obliterative and six were near-obliterative. Two patients had a history of prior urethroplasty. The length of the LMGs harvested ranged from 11 to 14 cm (mean, 12.8±1.03 cm). The preputial flaps available were from 1 to 1.5 cm in width (1.29±0.16 cm) and the desired length. Maximum urinary flow rate (Qmax) achieved ranged from 12 to 26 mL/s (mean, 20.46±3.71 mL/s) after 3 months. One patient needed a single direct visualized internal urethrotomy and another patient develop temporary superficial penile necrosis. The success rate was 90%.
Single-stage reconstruction of long, obliterative urethral strictures is a challenge. Since the introduction of the inner preputial and distal penile circular fasciocutaneous flap by Quartey [1] and McAninch [2], this hairless, adequately sized flap has been used for the treatment of urethral strictures even in circumcised patients [3]. Although the preputial flap is tubularized to substitute for the urethra, its surgical outcome is poor. Humby and Higgins in 1941 [4] introduced the buccal mucosal graft (BMG) for hypospadias, which was later rediscovered and popularized by Burger et al. in 1992 and is now being used for augmentation of the urethra with good outcomes [56789]. The management of long obliterative and near-obliterative urethral strictures remains challenging, however, with either staged urethroplasty or excision and substitution urethroplasty in a single-stage procedure as available options. Using an oral mucosal graft (OMG) alone for single-stage reconstruction of a long urethral segment is not possible, especially in the penile region. However, staged procedures are associated with increased morbidity, financial burden, and difficulty in psychosocial adjustment [10]. In addition, there is no surety of better outcome than with a single-stage procedure [11]. Therefore, staged procedures should be reserved for complex strictures after failed hypospadias repair, lichen sclerosus, and strictures complicated by fistula or abscess [9]. The principle of augmentation urethroplasty using an OMG and penile skin was first introduced by Morey [12] and later by Erickson et al. [13]. Here we report our initial experience with this technique.
This was a retrospective study of 10 patients who underwent this technique between January 2015 and June 2017. Ethical approval was obtained from the institutional ethics committee (approval number: IRB 2425/EC/MC/2016). All patients included in the study had narrow preputial skin owing to a previous history of circumcision and had long obliterative or near-obliterative anterior urethral strictures. Patients having lichen sclerosus et atrophicus, urethral abscess and fistula, short stricture and other comorbidities were excluded. Patients with buccal mucosa showing submucosal fibrosis or otherwise not suitable for grafting were also excluded.
Preoperative evaluation included clinical history, physical examination, urine culture, uroflowmetry (UFM), retrograde urethrogram (RGU), and voiding cystourethrogram (VCUG). Urethroscopy was done at the time of surgery. Urethras with a caliber <5 Fr but a patent lumen were labeled as near-obliterative urethral strictures.
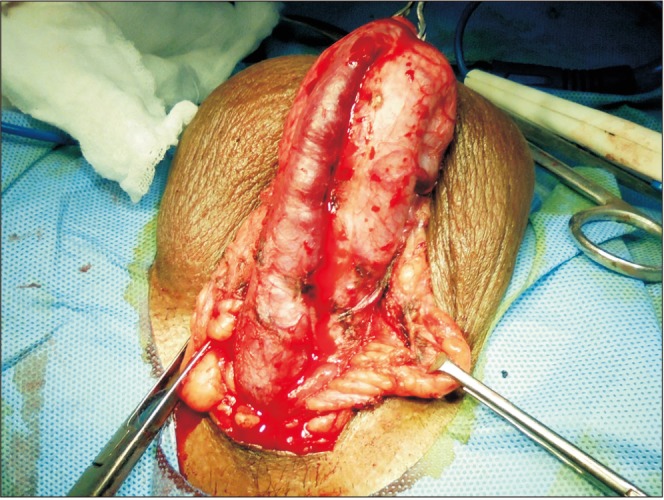
The procedure was performed on patients in the lithotomy position under general anesthesia. All cases were performed by a single experienced surgeon. A midline perineal and proximal scrotal incision was made depending on the location of the stricture. The incision was extended according to stricture length. Another circumferential, subcoronal incision was made and was deepened to the superficial layer of Buck's fascia. Subsequently, the urethra was dissected and the penis was pulled out from the perineal incision site (Fig. 1). In cases of obliterative strictures, the entire fibrosed segment was excised. Excision of urethral tissue was extended proximally and distally until approximately 1 cm of healthy urethra was exposed at both ends. In cases of a narrowed urethra, the urethra was dissected dorsolaterally from the corporal body and incised. This incision was extended in healthy proximal and distal urethra for 1 cm. After the urethral mucosa of the stricture segment was incised, if it was found to have a normal appearance, it was saved for augmentation. Otherwise, it was excised.
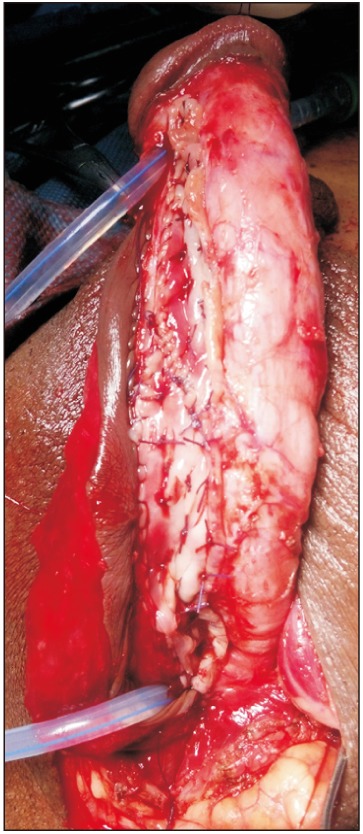
The lingual mucosal graft (LMG) and inner preputial flap were harvested simultaneously by two teams. For the LMG, harvesting was done by use of a standard technique [14]. The width was kept to about 1.5 to 2 cm and the length to about 2 cm longer than the stricture length. In cases in which graft length on one side was inadequate, another graft was taken from the opposite side of the tongue. Bleeding in the donor site bed was controlled with bipolar cautery and left open. The oral wound side was packed with adrenaline-soaked ribbon gauze for 3 to 5 hours. Graft defatting was done until all underlying fibrofatty tissue was completely removed and the glistening surface visualized. Multiple small incisions were made to make fenestrations in the graft to prevent collection between the graft and the bed. Subsequently, the graft was spread and quilted over the ventral surface of the corporal bodies (Fig. 2).
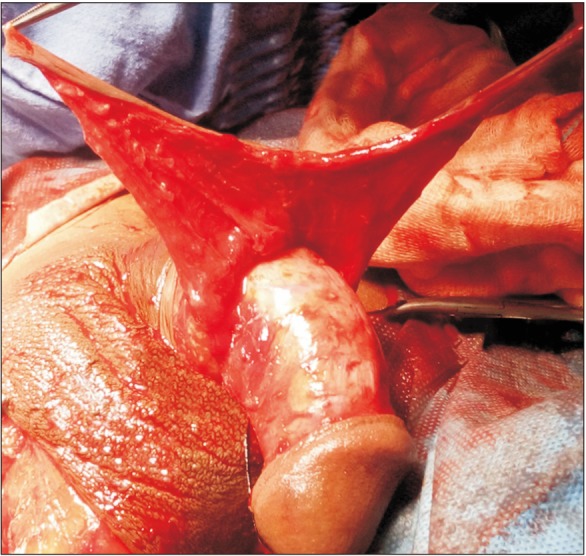
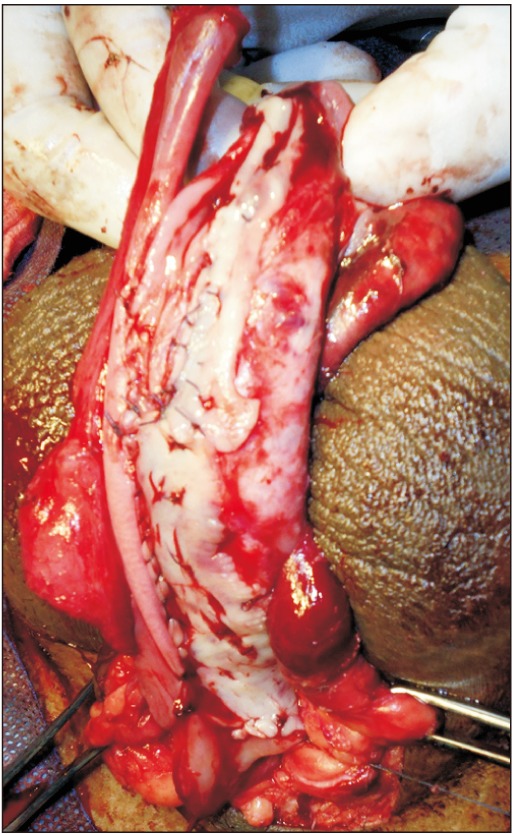
The preputial fasciocutaneous flap was developed by the McAninch technique. A circumcoronal incision was marked about 4 to 5 mm proximal to the glanular margin, followed by a second incision marked about 1 to 1.5 cm proximal to the first. A distal incision was deepened to the superficial layer of Buck's fascia. Once the proper plane of dissection was achieved, dissection was carried out circumferentially. This dissection was carried up to the root of the penis. Subsequently, a proximal circumferential incision was deepened through the thin superficial layer of dartos and then the dissection was done to create a space between the deeper and superficial layers of the dartos fascia. This maneuver helps to protect the subdermal vascular plexus of penile skin, thus avoiding necrosis. The second plane of dissection was also extended to the base of the penis. The preputial flap with its pedicle was divided ventrally in the midline (6 o'clock) down to the penoscrotal junction, thereby converting its circular configuration into a longitudinal strip (Fig. 3). After proper mobilization, the preputial flap was brought to the area of repair by the side of the penis in a tension-free manner. In cases of obliterative urethral strictures, the lateral edges of the LMG and preputial skin were sutured together, incorporating the tunica albuginea (Fig. 4) [5]. In cases of near-obliterative urethral strictures, the medial margin of the lingual mucosa was sutured to the medial margin of the laid-open urethra incorporating the tunica albuginea, so as to create the roof of neourethra (Fig. 5) [6]. By this we were able to achieve 26 to 30 mm (26 to 30 Fr) total breath of neourethral plate. Following this, one margin of vertically oriented preputial flap was sutured with the free edge of the laid-open urethra and another to the lateral margin of LMG so as to construct a urethral tube over a 14-Fr Foley catheter using 5–0 absorbable sutures. Then the degloved penile skin was retracted and the circumcoronal penile skin incision was closed. Bulbospongiosus muscle was approximated and the perineal wound was closed in layers after a negative-pressure suction drain was placed, which was removed after 48 hours or longer, once drainage was insignificant. The urethral catheter was removed at the end of 3 weeks.
The patients were followed up at 1, 3, and 6 months and then annually. UFM was done at every follow-up visit. If the patients had a maximum urinary flow rate (Qmax) <15 mL/s on UFM with evidence of stricture reappearance in RGU and VCUG or in endoscopy, then the procedure was labeled as a failure. Success was defined as no additional need for urethral instrumentation. Any direct visualized internal urethrotomy (DVIU) or repetitive dilatation or further surgical management for restricture was deemed a failure.
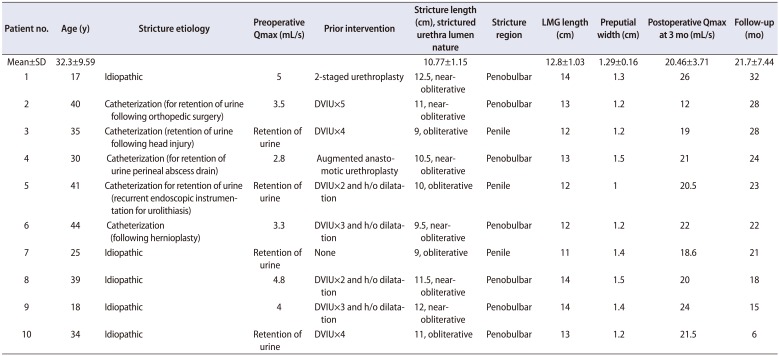
Table 1 shows the demographic data of the 10 patients who underwent neourethral reconstruction by the described technique. Most of the patients had a history of multiple endoscopic procedures. Seven patients had a history of ritual circumcision, whereas three had undergone circumcision in childhood for unknown reasons. In all patients, bilateral LMGs were harvested. The length of the harvested LMGs was matched to the stricture length. The preputial flaps raised were narrow in width and were of lengths matching the dorsal graft.
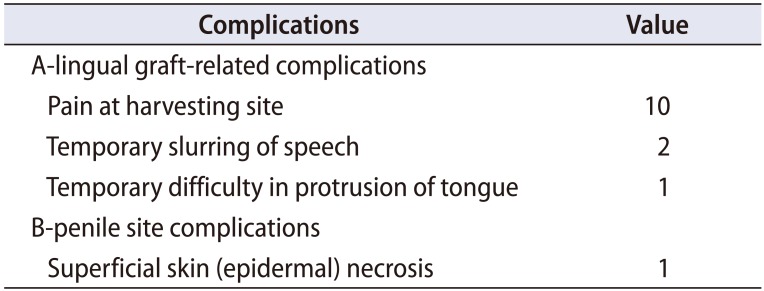
Table 2 shows the complications and morbidities associated with the procedures. OMG donor site morbidities were pain and slurring of speech, which subsided within 3 weeks without any treatment. One patient developed difficulty in protrusion of the tongue, which also resolved after proper exercise and speech therapy. One patient developed blackening and denudation of distal superficial penile skin that reepithelized completely within 3 weeks without any treatment.
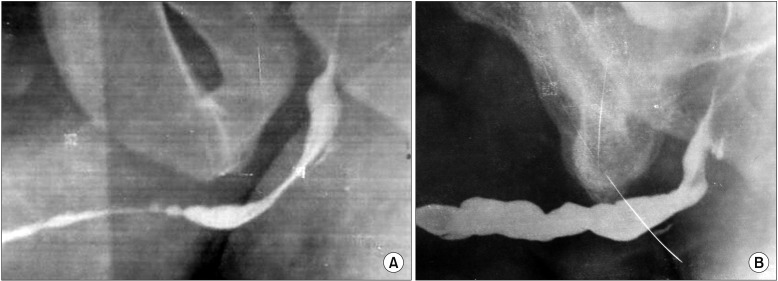
Follow-up time ranged from 6 to 32 months (mean, 21.7±7.44 months). The Qmax at 3 months after surgery varied from 12 to 26 mL/s. One patient developed narrowing of the distal bulbar anastomotic site for which DVIU was done. Subsequently, he voided well with a good stream. The preoperative urethrogram (Fig. 6A) shows the nearobliterative urethra and the postoperative RGU (Fig. 6B) after 2 years shows the good-caliber urethra.
Reconstruction of long urethral strictures is a challenging task, and the most favorable technique for repairing these strictures has not yet been adequately established. No study has clearly defined a preference for one tissue transfer technique over another for single-stage reconstruction of extensive anterior urethral strictures.
Before the use of OMGs for augmentation urethroplasty, preputial and penoscrotal flaps were being used for augmentation as well as substitution urethroplasty in cases of long obliterative or near-obliterative urethral strictures [1215161718192021]. A local penile skin flap has the advantage of quicker harvesting but lacks a definitive blood supply and has the disadvantage of hair-bearing skin [17].
On the other hand, the hairless, preputial-based pedicled flap has a good vascularized, long pedicle; therefore, it can be transported to nearly any part of the urethra [131621]. Developing a preputial island flap requires not only a long operative time but also expertise. Besides these individual advantages and disadvantages of local flaps, all skin-based flaps have the disadvantages of excoriation of skin on exposure to urine, restenosis, dribbling of urine, diverticula, and fistula formation [2322]. These can be reduced by incorporating a smaller area of skin in the urethral reconstruction and by closing the wound in multiple layers [221]. The technique is not without complications, however. The results of the initial two studies with tubularized penile or preputial-based flaps had success rates of about 58% [2023].
BMGs alone are not very helpful for single-stage repair of long obliterative and near-obliterative anterior urethras. Tubularization of BMGs has been shown to have a nearly 50% short-term restricture rate, the cause of which is hypothesized to be the unreliable blood supply the healing graft receives at its lateral edges [12]. However, a BMG can be used for short obliterative strictures by the augmented anastomotic technique [2425] or by use of a double-patched mucosal graft [726]. Here we used a LMG because it has less postoperative morbidity and more ease of harvesting compared with a BMG [27].
Only a few studies are available where long obliterative or near-obliterative urethral strictures were treated by using combined BMG and penile flaps. In these studies, the BMG was used for proximal and the penile skin flap for distal urethral plate augmentation with variable results (success rate, 38% to 88%) [242829]. Some authors constructed a neourethral tube using a dorsal BMG and a ventral penile flap in failed hypospadias and complex urethral strictures [1213]. Erickson et al. [13] used a combined tissue transfer technique in the treatment of urethral strictures. They excised the portion of the urethra with a width of <0.5 cm and significant fibrosis. The excised part was substituted by a BMG and this was augmented by a ventral penile flap. Where the plate was >0.5 cm wide, augmentation of the urethra was performed with a fasciocutaneous flap only. Their initial success rate was 64.3% [13].
In a particular situation, where preputial skin is deficient because of circumcision, a preputial skin tube cannot help in a single-stage correction of a long anterior urethral stricture. In these situations, the combination of preputial skin and oral mucosa can help in single-stage reconstruction of the urethra.
These may not only help in reducing skin flap–based complications but also allow the construction of neourethral tubes of adequate size. In the present study, we were able to treat long obliterative and near-obliterative urethral strictures in circumcised patients by using both LMGs and available inner preputial skin with good outcomes (90% success rate). In our technique, except for the circumcoronal incision, no other incision was made in the penile skin. The urethra was dissected and pulled out through the perineal or midscrotal incision, thus avoiding an overlap of suture lines. In the present study, the preputial flaps were narrow but with broad and long pedicles. Besides these maneuvers, suturing of both the LMG and the flap to the corporal bodies also helped in to prevent contracture as well as the formation of fistulas and diverticula [132021]. Necrosis of the penile skin proximal to the flap can result when the vascular supply of the subdermal plexus is compromised [20], and its incidence varies between 2% and 23% [32324]. The incidence in the present study was 10%. This can be reduced if the flap is raised in a proper plane with good hemostasis. Recurrence of stricture can be reduced by good hemostasis and meticulous anastomosis, keeping an adequate sized urethral lumen.
To the best of our knowledge, no other studies are available in which the neourethra was constructed by using dorsal LMG and ventral preputial skin flaps, particularly in circumcised patients. The limitations of the present study were the small number of cases and the limited period of follow-up.
References
1. Quartey JK. One-stage penile/preputial cutaneous island flap urethroplasty for urethral stricture: a preliminary report. J Urol. 1983; 129:284–287. PMID: 6834490.

2. McAninch JW. Reconstruction of extensive urethral strictures: circular fasciocutaneous penile flap. J Urol. 1993; 149:488–491. PMID: 8437252.

3. Carney KJ, McAninch JW. Penile circular fasciocutaneous flaps to reconstruct complex anterior urethral strictures. Urol Clin North Am. 2002; 29:397–409. PMID: 12371231.

5. Andrich DE, Mundy AR. Substitution urethroplasty with buccal mucosal free grafts. J Urol. 2001; 165:1131–1133. PMID: 11257653.
6. Pansadoro V, Emiliozzi P, Gaffi M, Scarpone P, DePaula F, Pizzo M. Buccal mucosa urethroplasty in the treatment of bulbar urethral strictures. Urology. 2003; 61:1008–1010. PMID: 12736025.

7. Sharma U, Yadav SS, Tomar V, Garg A. Single stage circumferential lingual mucosal graft urethroplasty in near obliterative bulbar urethra stricture: a novel technique. Urol Ann. 2016; 8:146–150. PMID: 27141182.

8. Datta B, Rao MP, Acharya RL, Goel N, Saxena V, Trivedi S, et al. Dorsal onlay buccal mucosal graft urethroplasty in long anterior urethral stricture. Int Braz J Urol. 2007; 33:181–186. discussion 186-7. PMID: 17488537.

9. Kulkarni S, Barbagli G, Sansalone S, Lazzeri M. One-sided anterior urethroplasty: a new dorsal onlay graft technique. BJU Int. 2009; 104:1150–1155. PMID: 19388990.

10. Moradi M, Moradi A. Urethroplasty for long anterior urethral strictures: report of long-term results. Urol J. 2006; 3:160–164. PMID: 17559033.
11. Andrich DE, Greenwell TJ, Mundy AR. The problems of penile urethroplasty with particular reference to 2-stage reconstructions. J Urol. 2003; 170:87–89. PMID: 12796651.

12. Morey AF. Urethral plate salvage with dorsal graft promotes successful penile flap onlay reconstruction of severe pendulous strictures. J Urol. 2001; 166:1376–1378. PMID: 11547078.

13. Erickson BA, Breyer BN, McAninch JW. Single-stage segmental urethral replacement using combined ventral onlay fasciocutaneous flap with dorsal onlay buccal grafting for long segment strictures. BJU Int. 2012; 109:1392–1396. PMID: 21880103.

14. Simonato A, Gregori A, Lissiani A, Galli S, Ottaviani F, Rossi R, et al. The tongue as an alternative donor site for graft urethroplasty: a pilot study. J Urol. 2006; 175:589–592. PMID: 16407002.

15. Orandi A. One-stage urethroplasty. Br J Urol. 1968; 40:717–719. PMID: 4883846.
16. Duckett JW. The island flap technique for hypospadias repair. Urol Clin North Am. 1981; 8:503–512. PMID: 7324317.

17. Mitre AI, Arap S, Lucon AM. Preputial island flap in extensive urethral stricture repair. World J Urol. 1992; 10:94–99.

18. Mundy AR, Stephenson TP. Pedicled preputial patch urethroplasty. Br J Urol. 1988; 61:48–52. PMID: 3342301.

19. Buckley J, McAninch J. Distal penile circular fasciocutaneous flap for complex anterior urethral strictures. BJU Int. 2007; 100:221–231. PMID: 17552974.

20. McAninch JW, Morey AF. Penile circular fasciocutaneous skin flap in 1-stage reconstruction of complex anterior urethral strictures. J Urol. 1998; 159:1209–1213. PMID: 9507836.

21. Atan A, Tuncel A, Balcı M, Aslan Y, Köseoğlu E, Erkan A. Penile fasciocutaneous flap urethroplasty in long segment urethral stricture. Ulus Travma Acil Cerrahi Derg. 2014; 20:427–431. PMID: 25541922.

22. Olajide AO, Salako AA, Aremu AA, Eziyi AK, Olajide FO, Banjo OO. Complications of transverse distal penile island flap: urethroplasty of complex anterior urethral stricture. Urol J. 2010; 7:178–182. PMID: 20845294.
23. Elbakry A. Complications of the preputial island flap-tube urethroplasty. BJU Int. 1999; 84:89–94. PMID: 10444131.

24. Dubey D, Srivastava A, Kapoor R, Kumar A, Bhandari M, Mandhani A. Single stage reconstruction of complex anterior urethral stricture. Indian J Urol. 2001; 17:145–151.
25. Guralnick ML, Webster GD. The augmented anastomotic urethroplasty: indications and outcome in 29 patients. J Urol. 2001; 165:1496–1501. PMID: 11342904.

26. Palminteri E, Manzoni G, Berdondini E, Fiore FD, Testa G, Poluzzi M, et al. Combined dorsal plus ventral double buccal mucosa graft in bulbar urethral reconstruction. Eur Urol. 2008; 53:81–89. PMID: 17583417.

27. Chauhan S, Yadav SS, Tomar V. Outcome of buccal mucosa and lingual mucosa graft urethroplasty in the management of urethral strictures: a comparative study. Urol Ann. 2016; 8:36–41. PMID: 26834399.

28. Wessells H, Morey AF, McAninch JW. Single stage reconstruction of complex anterior urethral strictures: combined tissue transfer techniques. J Urol. 1997; 157:1271–1274. PMID: 9120918.

29. Berglund RK, Angermeier KW. Combined buccal mucosa graft and genital skin flap for reconstruction of extensive anterior urethral strictures. Urology. 2006; 68:707–710. discussion 710. PMID: 17070336.

Fig. 2
Combined substitution urethroplasty. Lingual mucosal graft (LMG) quilted over ventral surface of corporal bodies and one side edge of longitudinally oriented preputial flap sutured to the right side margin of LMG incorporating tunica albuginea.
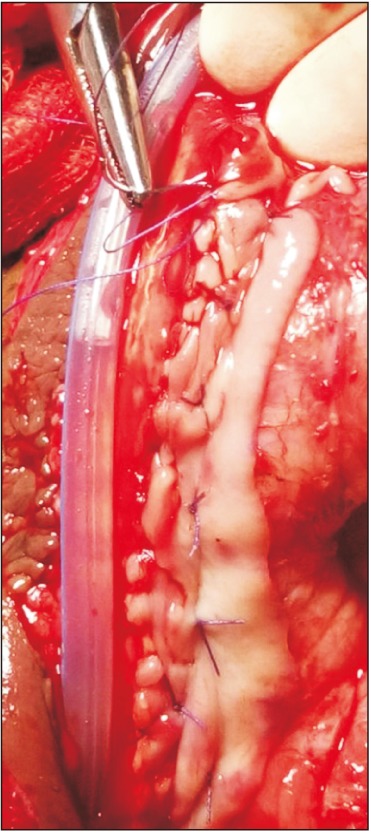
Table 1
Patient and stricture characteristics




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download