Abstract
Purpose
We investigated factors affecting testosterone recovery after androgen deprivation therapy (ADT) withdrawal in patients with prostate cancer.
Materials and Methods
The medical records of patients who underwent radical prostatectomy with ADT were retrospectively reviewed. In all, 221 patients were included in the analysis. Testosterone recovery was defined as supra-castration (SC) (testosterone levels in serum >50 ng/dL) or out of hypogonadism (OH) (>300 ng/dL) after ADT withdrawal. Kaplan-Meier analyses were used to estimate testosterone recovery after ADT cessation. Cox regression analyses were used to determine the factors affecting the recovery of testosterone.
Results
After ADT, 206 patients (93.2%) recovered to the SC level and 122 patients (55.2%) recovered to the OH level. Patients treated with ADT for ≤18 months recovered to OH in a mean of 6.8 months (74.6%), but patients treated with ADT for >18 months recovered in a mean of 9.7 months (27.5%). In multivariate analyses, age (hazard ratio [HR], 0.915; p<0.001), serum level of sex hormone-binding globulin (SHBG) (HR, 1.015; p=0.002), initial testosterone level (HR, 1.002; p=0.002), and ADT duration (HR, 0.915; p<0.001) were associated with recovery to the OH level after ADT withdrawal, and hypertension (HR, 0.697; p=0.029) and duration of ADT (HR, 0.979; p=0.012) were significantly associated with recovery to SC.
Prostate cancer is one of the most common cancers in men [1]. Androgen deprivation therapy (ADT) is a mainstay treatment of metastatic or advanced prostate cancer and has been shown to improve overall survival [23]. However, luteinizing hormone-releasing hormone analogue (LHRHa) therapy is associated with side effects, such as hot flashes, cardiovascular disease, metabolic syndrome, fatigue, anemia, depression, loss of libido, sexual dysfunction, and osteoporosis. Some of these are associated with increased mortality or degrade the quality of life [45]. Some adverse events are associated with decreased testosterone due to ADT and can be resolved by the recovery of testosterone after ADT discontinuation [3]. However, the time course of the recovery of testosterone after ADT withdrawal and the factors affecting recovery have not been well documented. A clear understanding of the timing and extent of testosterone recovery will provide better insight into how long the side effects of androgen suppression may persist after its discontinuation [6]. In our current study, we determined testosterone recovery and evaluated the factors associated with testosterone recovery after the withdrawal of ADT in patients with prostate cancer.
The medical records of 7,427 prostate cancer patients who underwent radical prostatectomy (RP) from January 2001 to December 2014 at Asan Medical Center were retrospectively reviewed, and 745 patients who received neoadjuvant, adjuvant, or salvage ADT were selected. Patients with continuous use, lost to follow-up, for whom it had not been more than 6 months since withdrawal of ADT, who did not have male hormone follow-up within 1 year of discontinuation of ADT, who did not have initial testosterone value, and who received neoadjuvant chemotherapy were excluded. The final analysis included 221 patients. We reviewed the data, including patient characteristics, tumor characteristics, ADT medicine types, and serum lab results.
Testosterone recovery was divided into two defined groups, supra-castration (SC) (testosterone levels in serum >50 ng/dL) and out of hypogonadism (OH) (>300 ng/dL). To avoid daily fluctuation, the testosterone levels used were measured in the morning. The initial testosterone values were those measured before the patients had undergone RP and begun ADT. Serum levels of testosterone were taken from all patients before ADT, and it was measured regularly after ADT cessation. Follow-up duration was defined as the time until the patient's last outpatient visit for measuring testosterone or until starting other hormone therapy due to cancer progression. All patients were available to analyze testosterone recovery from the date at which the ADT reactive time had ended. The duration of ADT-use was counted from the date of LHRHa administration for the beginning of withdrawal from ADT, which included each reaction period of the agents. At 1 month, 3 months, and 6 months of receiving LHRHa agents, the duration of administration of the ADT would be 1 month, 3 months, and 6 months after the agent was injected, respectively.
We used standard descriptive statistics to characterize the patients. Quantitative variables are expressed as means with a 95% confidence interval (CI). To determine the rate of male hormone recovery after ADT withdrawal, absolute recovery rates were obtained for each period (6, 12, 24, 36 months). Patients who started ADT again at a level of serum hormone levels below 300 were considered to be unrecovered when absolute recovery rates were obtained. However, Kaplan-Meier analysis was used to estimate the cumulative incidence of testosterone recovery after discontinuation of ADT and to generate recovery curves. Univariate and multivariate analyses were used to determine the factors affecting testosterone recovery using the Cox regression method. We generated receiver operating characteristic (ROC) curves to determine the performance of testosterone recovery according to the duration of ADT administration; we determined the time for the maximal sensitivity and specificity. Statistical analyses were performed by IBM SPSS Statistics for Windows ver. 20.0 (IBM Co., Armonk, NY, USA), and p<0.05 was considered significant. This study was approved by the Institutional Review Board of Asan Medical Center (approval number: 2015-0322).
The median age at the time of RP was 64 years old (mean±standard deviation [SD], 63.4±7.1 years old), and the median age at the beginning of ADT was 66 years (64.7±7.1 years). The median duration of ADT was 17 months (15.7±10.0 months), and the median follow-up duration after withdrawal of ADT was 16 months (22.1±16.0 months). Out of 170 patients (76.9%) who underwent salvage (after biochemical recurrence) treatment, 25 (11.3%) and 26 (11.8%) used neoadjuvant and adjuvant therapy, respectively. The LHRHa agonist agents used were goserelin (38.0%), leuprorelin (40.7%), and triptorelin (6.3%), and 33 patients (14.9%) changed treatment agents during use. The mean initial testosterone level measured before RP and ADT administration was 465.3±166.8 ng/dL (95% CI, 443.2–487.4), and hypogonadism (testosterone levels in serum <300 ng/dL) was found in 19 (8.6%) of 221 patients before ADT. The sex hormone-binding globulin (SHBG) value was measured in 168 patients (76%), and the mean value was 60.6±23.8 nmol/L. The pathologic Gleason score was less than seven points in 101 (45.7%), eight points in 56 (25.3%), and more than nine points in 64 (29.0%); 46 (20.8%), 98 (44.3%), and 77 patients (34.8%) had stage T2, T3a, and above T3b, respectively. Antiandrogen was used in 148 of 221 patients (67.0%) with ADT (Table 1).
After ADT, 217 patients (98.2%) had testosterone levels below the castration level (testosterone levels in serum <50 ng/dL), and the median time to castration was 2 months (3.3±4.5). In all, 206 patients (93.2%) reached the SC level after ADT discontinuation, and the median time taken to this level was 7 months (7.9±5.7 months); 122 (55.2%) patients recovered to the OH level, and the median recovery period was 9 months (12.1±10.5 months). The mean follow-up period was 25.1±16.7 months in patients recovering to OH level. And the mean follow-up period was 18.4±14.3 months in patients who remained below the OH level. The absolute recovery rates of OH were 47 (21.3%), 82 (37.1%), 105 (47.5%), and 117 (52.9%) at 6, 12, 24, and 36 months after discontinuation of ADT, respectively. In the Kaplan-Meier analysis for estimating the cumulative incidence of OH, we could expect recovery rates of 21.7%, 40.4%, 58.1%, and 74.4% at 6, 12, 24, and 36 months, respectively (Fig. 1). At the time of discontinuation of hormone therapy, the male hormones averaged 14.9±20.8 ng/dL (95% CI, 12.1–17.7), 171.9±190.3 ng/dL (95% CI, 146.2–197.7) at 6 months, 260.0±190.6 ng/dL (95% CI, 231.1–289.0) at 12 months, and 230.0±182.1 ng/dL (95% CI, 294.8–268.1) at 24 months after discontinuation of ADT, respectively (Fig. 2).
The ROC curve was used to compare OH according to the duration of ADT. The sensitivity (67.7%) and specificity (76.5%) had reached maximal values at 18.5 months for OH, and it was able to be distinguished two groups significantly. Kaplan-Meier curves analyzing of testosterone recovery greater than OH in the established groups according to the duration of ADT administration (≤18 months vs. >18 months) are presented in Fig. 3. Of 130 patients treated with ADT for 18 months or less, 97 (74.6%) had testosterone recovery greater than OH level, compared to 25 of 91 patients (27.5%) treated with ADT 19 months or more, and the mean times for recovery were 6.8 months and 9.7 months (p<0.001), respectively. Fig. 4 shows the difference in recovery (>OH), comparing testosterone levels for each period after ADT use between the two groups.
Cox analyses were performed to determine the factors affecting testosterone recovery after ADT withdrawal. In univariate analyses, in patients without hypertension (HR, 0.732; 95% CI, 0.555–0.966; p=0.028), higher serum levels of SHBG (HR, 1.008; 95% CI, 1.001–1.014; p=0.028) and shorter duration of ADT (HR, 0.974; 95% CI, 0.958–0.989; p=0.001) was significantly associated with recovery to SC. In multivariate analyses, in patients without hypertension (HR, 0.697; 95% CI, 0.504–0.964; p=0.029) and shorter duration of ADT (HR, 0.979; 95% CI, 0.962–0.995; p=0.012) were significantly associated with recovery to the SC level (Table 2). Univariate analyses showed that patients who were younger at the time of ADT application (HR, 0.963; 95% CI, 0.939–0.987; p=0.003), who without diabetes (HR, 0.545; 95% CI, 0.325–0.912; p=0.021), who without hypertension (HR, 0.676; 95% CI, 0.468–0.976; p=0.037), who had a lower body mass index (HR, 0.931; 95% CI, 0.887–0.979; p=0.005), higher serum SHBG levels (HR, 1.014; 95% CI, 1.006–1.022; p=0.001), higher initial testosterone levels (HR, 1.002; 95% CI, 1.000–1.003; p=0.006), and shorter duration of ADT (HR, 0.936; 95% CI, 0.915–0.958; p<0.001); and who did not use anti-androgens with ADT (HR, 0.524; 95% CI, 0.360–0.761; p=0.001) were more likely to recover to OH. In multivariate analyses, age at application of ADT (HR, 0.915; 95% CI, 0.885–0.946; p<0.001), higher serum SHBG level (HR, 1.015; 95% CI, 1.005–1.025; p=0.002), higher initial testosterone (HR, 1.002; 95% CI, 1.001–1.004; p=0.002), and duration of ADT (HR, 0.915; 95% CI, 0.888–0.942; p<0.001) influenced OH (Table 3).
The timing of the recovery of testosterone levels while maintaining prostate-specific antigen (PSA) nadir levels after the completion of ADT therapy in advanced prostate cancer patients is not well known. Several studies have investigated the recovery of testosterone after the discontinuation of ADT. Murthy et al. [7] reported the evaluation of the time-course of testosterone recovery after ADT in prostate cancer. The average duration of ADT administration in that study was 116 days (95% CI, 54–194). After ADT, many patients had normal testosterone levels (>6 nmol/L) until 6 weeks, and testosterone recovery reached 35% at 12 weeks (mean, 11.4 nmol/L), 85% at 18 weeks, and 89% at 24 weeks. The median time for testosterone recovery was 13 weeks. In another study [8], 15 patients who had received 4 or more years (range, 48 to 110 months) of continuous ADT for prostate cancer were reviewed, and the proportion of patients who remained at the castration level after ADT discontinuation was estimated. Eight patients (53%) maintained castration levels (<50 ng/dL), six (40%) had subnormal levels (≥50 ng/dL and <240 ng/dL), and only one patient (7%) had normal levels (≥240 ng/dL). The mean follow-up duration was 31 months (range 19 to 51 months) after ADT cessation.
In a prospective study of testosterone recovery reported by Yoon et al. [6], 36.5 months after the discontinuation of ADT, 71.2% returned to normal levels and 93.2% returned to at least the SC level. Nejat et al. [9], who performed a prospective study on testosterone recovery after discontinuation in 68 patients who received ADT, showed testosterone recovery rates of 28%, 48%, and 74% at 3 months, 6 months, and 12 months, respectively (≥270 ng/dL). They also found that testosterone normalization (270–1,070 ng/dL) took longer in patients who used it for more than 24 months in the period of ADT use (p=0.0034).
The rate of recovery in our current study was lower than the rate in previous studies because of the difference in baseline between testosterone recovery definitions. Although this does not align with previous studies, it may be important to use hypogonadism as a testosterone recovery criterion. Decreases in testosterone are associated with side effects, and some side effects are resolved by testosterone recovery [4]. Therefore, setting criteria for testosterone recovery from hypogonadism may have clinical significance and be helpful for patients.
In our present study, time in ADT also affected testosterone recovery. Using the maximal value (18.5 months) obtained from the ROC curve, we showed the difference in testosterone recovery rate and period of testosterone recovery in the group in ADT for 18 months or less and for the group in it for more than 18 months. Planas et al. [10], who studied luteinizing hormone and testosterone recovery after ADT interruption in 40 patients with long-term ADT, compared a group treated for 60 months or more and a group treated for less than 60 months, and the duration of ADT was the only factor affecting testosterone recovery. The mean recovery durations were 29.3 months (95% CI, 19.6–39.1) and 14.5 months (95% CI, 6.5–22.6), respectively (p=0.029). Other studies have shown a similar result, that the duration of ADT administration has statistically significant effects on testosterone recovery [679].
By multivariate analyses, younger at the time of ADT application and higher serum basal SHBG levels were found to be influential for reaching OH after ADT. Long-term treatment with LHRHa agonists was strongly inhibitive of testosterone production in Leydig cells. ADT against prostate cancer causes changes in testicular histology by its severe atrophy of the seminiferous tubules and Leydig cells [11]. Many previous studies have reported the clear benefit of androgen ablation by LHRHa in reducing testosterone to the castration level for the treatment of advanced prostate cancer [1213]. However, old age and maximal androgen blockage reduced testosterone recovery. Pedraza and Kwart [14] reported on four elderly patients (mean age, 70 years old) who received long-term LHRHa treatment. All four patients remained at the castration level 3 years after ADT cessation with undetectable PSA levels. As reported by Bong et al. [8], the castration level in their study population was maintained at 78% and 17% when ADT administration was compared between patients of age older than 70 years old vs. 70 years old and younger, respectively. SHBG levels serve as a steroid reservoir. In a normal man's blood there is only about 2% free testosterone (unbound), with 44% bound to SHBG and 54% to other proteins or albumin [15]. Therefore, SHBG in serum may play the role of a testosterone reservoir, as well as being beneficial to hormone levels when concentrations are low. This may be the cause of SHBG's role in the recovery of testosterone.
There were some limitations to this study of note. First, this study was conducted retroactively. In addition, there was a difference in the duration of testosterone measurement and the total follow-up period in each patient. Second, this study was limited to patients who had undergone RP. Data from prostate cancer patients who had radiation therapy with ADT or ADT alone were missing, and the effects of surgery were not evaluated. Third, we could not evaluate the relationship between testosterone recovery and side effects of ADT use. We could not identify side effects in ADT patients and could not determine whether the side effects were affected by testosterone recovery after ADT cessation. Future studies should be planned as a prospective study. Therefore, the testosterone measurement period should be the same after the ADT interruption, and the period of maintaining the OH level should also be included. The relationship between testosterone recovery and functional recovery or resolution of side effect should be studied.
Age at ADT administration, SHBG level, initial testosterone level, and duration of ADT are factors associated with testosterone recovery in prostate cancer patients treated with ADT in whom therapy has been stopped. Patients treated with ADT for 18 months or less recover their testosterone levels more and faster after the withdrawal of ADT.
Figures and Tables
Fig. 1
(A) The absolute recovery rates of testosterone recovery to out of hypogonadism (>300 ng/dL) after androgen deprivation therapy (ADT) withdrawal. (B) The Kaplan-Meier analysis for estimating the cumulative incidence of out of hypogonadism (>300 ng/dL) after ADT withdrawal.

Fig. 2
Mean testosterone recovery level (>300 ng/dL) by duration after androgen deprivation therapy (ADT) withdrawal. CI, confidence interval.

Fig. 3
Cumulative rates of out of hypogonadism after androgen deprivation therapy (ADT) by ADT duration (blue: ≤18 months, red: >18 months).

Fig. 4
Mean testosterone recovery level (>300 ng/dL) by duration after androgen deprivation therapy (ADT) withdrawal.

Table 1
Clinical and pathological characteristics of the study cohort

Table 2
Univariate and multivariate Cox proportional hazards regression analyses of factors significantly associated with testosterone recovery to supra-castration (>50 ng/dL)
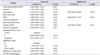
Table 3
Univariate and multivariate Cox proportional hazards regression analyses of factors significantly associated with testosterone recovery to out of hypogonadism (OH) (>300 ng/dL)
References
1. Klap J, Schmid M, Loughlin KR. The relationship between total testosterone levels and prostate cancer: a review of the continuing controversy. J Urol. 2015; 193:403–413.

2. Kamada S, Sakamoto S, Ando K, Muroi A, Fuse M, Kawamura K, et al. Nadir testosterone after long-term follow-up predicts prognosis in patients with prostate cancer treated with combined androgen blockade. J Urol. 2015; 194:1264–1270.

3. Nguyen PL, Alibhai SM, Basaria S, D'Amico AV, Kantoff PW, Keating NL, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015; 67:825–836.

4. D'Amico AV, Denham JW, Crook J, Chen MH, Goldhaber SZ, Lamb DS, et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007; 25:2420–2425.
5. Salonen AJ, Taari K, Ala-Opas M, Viitanen J, Lundstedt S, Tammela TL, et al. Advanced prostate cancer treated with intermittent or continuous androgen deprivation in the randomised Finn Prostate Study VII: quality of life and adverse effects. Eur Urol. 2013; 63:111–120.
6. Yoon FH, Gardner SL, Danjoux C, Morton G, Cheung P, Choo R. Testosterone recovery after prolonged androgen suppression in patients with prostate cancer. J Urol. 2008; 180:1438–1443. discussion 1443-4.

7. Murthy V, Norman AR, Shahidi M, Parker CC, Horwich A, Huddart RA, et al. Recovery of serum testosterone after neoadjuvant androgen deprivation therapy and radical radiotherapy in localized prostate cancer. BJU Int. 2006; 97:476–479.

8. Bong GW, Clarke HS Jr, Hancock WC, Keane TE. Serum testosterone recovery after cessation of long-term luteinizing hormone-releasing hormone agonist in patients with prostate cancer. Urology. 2008; 71:1177–1180.

9. Nejat RJ, Rashid HH, Bagiella E, Katz AE, Benson MC. A prospective analysis of time to normalization of serum testosterone after withdrawal of androgen deprivation therapy. J Urol. 2000; 164:1891–1894.

10. Planas J, Celma A, Placer J, Cuadras M, Regis L, Gasanz C, et al. Hormonal response recovery after long-term androgen deprivation therapy in patients with prostate cancer. Scand J Urol. 2016; 50:425–428.

11. Johansen TE, Ogreid P, Kjellevold K, Blom P. Testicular histology after treatment with LH-RH analogue for carcinoma of the prostate. Br J Urol. 1990; 65:376–378.

12. Crook JM, O'Callaghan CJ, Duncan G, Dearnaley DP, Higano CS, Horwitz EM, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012; 367:895–903.

13. van den Bergh RC, van Casteren NJ, van den Broeck T, Fordyce ER, Gietzmann WK, Stewart F, et al. Role of hormonal treatment in prostate cancer patients with nonmetastatic disease recurrence after local curative treatment: a systematic review. Eur Urol. 2016; 69:802–820.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download