Abstract
Purpose
To describe the salvage radical prostatectomy (sRP) experience in patients presenting with recurrent, clinically localized prostate cancer after multiple failed local treatments.
Materials and Methods
Among the 251 sRP performed during 2000–2016, 11 patients had failed multiple local therapies. We describe baseline clinical characteristics at primary cancer diagnosis and prior to sRP, surgical information, complications and oncological outcomes.
Results
The mean±standard deviation age at sRP was 65±5 years and the median (interquartile range) serum prostate-specific antigen (PSA) level was 2 (1.3) ng/mL. The most common first and subsequent treatments were radiotherapy and cryotherapy, respectively, with median time of 24 months from the last local treatment. The median operative time was 180 minutes and median estimated blood loss was 750 mL. Five (45.5%) patients underwent additional procedures during sRP for pre-operative morbidity from prior treatments (rectourethral fistula, urethral stricture, incontinence). Post-operative complications requiring invasive intervention occurred in 7 (63.6%) patients. Over a median follow-up of 29 (12–96) months, 10 of the 11 men (90.9%) achieved an undetectable PSA in after sRP. Three of these men with an initially undetectable PSA level experienced biochemical recurrence; the remaining 7 are without evidence of disease. Overall, no local recurrence or systemic metastasis was identified at last follow-up.
Conclusions
sRP is technically feasible and offers durable cancer control in patients with recurrent prostate cancer despite having undergone multiple prior attempts at cure. These patients experience higher rates of post-operative complications and such patients must be appropriately counseled regarding the potential risks and benefits.
About one third of men with newly diagnosed prostate cancer (PCa) chose radiation therapy (RT) as a primary treatment option [1]. Failure after RT is not uncommon and based on the type and dose of RT, duration of follow-up and the definition used, reported failure rates can reach as high as 60% [23]. Approximately 20% to 30% of men with biochemical recurrence (BCR) after RT are likely to harbor residual cancer within their prostate and may potentially benefit from salvage treatments with curative intent [45]. However, the Cancer of the Prostate Strategic Urological Research Endeavor (CaPSURE) database found 94% of men with BCR after RT were treated with androgen deprivation therapy (ADT) alone which is not curative and associated with significant systemic morbidities [6]. Less than 7% patients received salvage curative treatments such as RT, radical prostatectomy or ablative therapies [7]. Salvage radical prostatectomy (sRP) appears to offer durable oncological outcomes but the complication rates and the associated morbidity remains high [8]. Because of the technical demands of sRP, as well as the morbidity associated with this procedure, alternative salvage treatments have been developed. These primarily include salvage whole gland or partial gland ablation with the most and commonly used energies for ablation being thermal based—cryotherapy and high intensity focused ultrasound [910].
Failure rate after salvage ablation treatment for radiation-recurrent PCa is as high as 74% depending on both pre-radiation and pre-salvage ablation clinical features [6]. A small proportion of patients with post salvage therapy failure have local recurrence only which may be amenable to second salvage treatment. Higher utilization of non-surgical (radiation/ablation) initial salvage treatments for recurrent PCa after primary RT has resulted in patients presenting with local recurrence after more than one primary local treatment. sRP can be considered as a treatment option in this subset of patients after careful evaluation of local extent of disease and to confirm no evidence of metastasis. These patients present with a high level of surgical complexity due to obliterated surgical planes and complications (recto urethral fistula, urethral stricture disease, incontinence) resulting from the multiple prior local treatments. We report our surgical experience of sRP in patients with recurrent PCa following multiple prior local treatments.
Between 2000 and 2016, we performed sRP for 251 patients with biopsy proven clinically localized recurrent PCa. Following an exempt from approval of Institutional IRB (approval number: 16-1159), we queried the institutional prospectively maintained PCa database to identify 11 patients who received more than one local therapy prior to sRP (Table 1). Ten patients received 2 prior local treatments and 1 patient had 3 treatments prior to sRP. The mean±standard deviation (SD) age at primary cancer diagnosis was 57±6 years and the median (interquartile range, IQR) prostate specific antigen was 5.3 (1.6) ng/mL. The initial Gleason score was 6 (3+3; Gleason grade group 1) in 6 patients and 7 (3+4; Gleason grade group 2) in 5 patients. The primary diagnosis and treatment was offered between 1992 and 2006 and all the first treatments were radiation-based including external beam radiotherapy, brachytherapy or a combination of both. The second treatment was offered within a median time period of 48 (12–180) months from the first treatment. The most common salvage local treatment offered after failed radiation was whole gland cryoablation noted in 9 patients.
Prior to sRP, all the patients underwent transrectal ultrasound guided prostate biopsies confirming persistent prostate cancer; all biopsies were reviewed by our pathologist. Magnetic resonance imaging (MRI) of the prostate was performed in all patients to evaluate the loco-regional extent of disease. None of the patients had radiographic evidence of metastasis prior to sRP. All patents underwent sRP with extended bilateral pelvic lymph node dissection. Carefully selected patients also underwent additional surgical procedures during sRP to treat complications from previous salvage treatments including urinary incontinence, urethral stricture, and rectourethral fistula. Operative details (type, duration, intraoperative complications and blood loss), post-operative complications and their management were noted.
Following sRP patients underwent periodic evaluations including prostate-specific antigen (PSA) measurements. BCR was defined as a PSA >0.1 ng/mL. Radiographic evaluation (computed tomography, MRI, and positron emission tomography scans) were performed in patients with BCR to evaluate for local and/or systemic disease. Last follow-up information was obtained from the electronic medical record and telephonic enquiry was made to patients whose last follow-up was more than 12 months. Details of local recurrence, systemic metastasis and progression to castration resistant state were noted. In case of death, the cause of death was determined by chart review or death certificate. We reported means, medians, and interquartile ranges for continuous variables. Frequencies and proportions were reported for categorical variables.
The mean±SD age at sRP was 65±5 years and the median (IQR) PSA was 2 (1.3) ng/mL (Table 2). The median time of the sRP from the last local treatment was 24 (12–72) months. Prior to sRP, Gleason score ≥7 was noted in 10 (90.9%) patients and pre-sRP imaging identified extraprostatic extension (EPE) and seminal vesicle invasion (SVI) in 45.5% and 27.3%, respectively. Six (54.5%) patients received hormonal treatment prior to sRP.
Other clinical problems noted prior to sRP were—one patient had rectourethral fistula and 2 patients had urethral stricture as complication of the prior salvage cryoablation. Five patients reported stress urinary incontinence with 2 of these men having severe incontinence. All the patients had erectile dysfunction prior to sRP.
sRP with extended bilateral pelvic lymphadenectomy was performed as an open procedure in 9 patients and robot assisted procedure in 2 patients. The median operative time was 180 minutes and the estimated blood loss (EBL) was 750 mL. Blood transfusion was required in one patient. In addition to sRP, surgical correction of the pre-existing clinical problems was performed in five men. Two patients with severe urinary incontinence were treated with artificial urinary sphincter and sling procedure, respectively. Two patients with urethral stricture underwent dilatation of the narrowed segment. Bilateral ureteric stenting and repair of the fistulous tract was done in one patient with rectourethral fistula from previous salvage cryoablation. The median (range) hospital stay following sRP was 2 (1–30) days.
The final histopathology of the sRP specimen revealed Gleason ≥7 cancer in 7 (63.6%) patients, cancer was not gradable due to therapy related changes in the remaining 4 (36.4%) patients. The pathological stage was >pT2 in 8 (72.7%) patients. The rates of positive surgical margin, EPE and SVI were 55%, 55%, and 36% respectively. Lymph nodes were positive for cancer in 3 (27.3%) patients.
One patient had rectal injury during sRP that was managed with primary closure of the rectum and a diverting colostomy. However, he developed a rectourethral fistula in the post-operative period that was successfully managed with fistulectomy, closure of the rectal defect, perineal urethroplasty, and an interposition muscular graft. Anastomotic urine leak was noted in 4 patients—two of these patients were managed with prolonged catheterization and the remaining two required prolonged catheterization plus bilateral percutaneous nephrostomies for temporary urinary diversion; in all 4 the anastomotic leak healed at a median of 6 (4–12) weeks after sRP. Anastomotic stricture (bladder neck contracture) was noted in 4 patients out of whom one was following a prior anastomotic leak. These men required multiple endoscopic incisions of the bladder neck. None of these 4 men recovered continence. Two of these men had mild-moderate incontinence managed with pads. One patient with intractable anastomotic stricture and urinary incontinence underwent continent cutaneous urinary diversion and augmentation cystoplasty and the other failed management with an artificial urinary sphincter and later required cystectomy and ileal conduit diversion for pubovesical fistula and intractable incontinence [9]. In the remaining 7 men without anastomotic stricture, 2 patients achieved complete continence and were pad free. Three had stress urinary incontinence managed with pads, and two were completely incontinent.
The median follow-up after sRP was 29 (12–96) months. Post operative undetectable PSA (<0.05 ng/mL) was achieved in 10 (90.9%) patients and subsequently BCR (PSA ≥0.1 ng/mL) was noted in 3 (27.3%) patients. One patient with BCR died from non-prostate cancer causes. No local recurrence or metastases were noted in the remaining 10 surviving patients at last follow-up.
Table 3 briefly summarizes each case detail.
Radiotherapy is one of the standard primary treatments for prostate cancer. In spite of advances in imaging guidance and conformal techniques, up to 60% of these patients can have BCR in the 10 year follow-up. While many of these men will fail systemically, a portion of these men have local recurrence only and are therefore potentially eligible for additional local therapy with curative intent. Without treatment, these patients are likely to develop symptomatic local progression and systemic metastasis [10]. ADT is the most commonly employed salvage treatment in radiation recurrent prostate cancer but is not curative. sRP offers excellent cancer control in men with clinically localized, radiation recurrent prostate cancer, but surgery is often not chosen as a salvage treatment considering the technical challenges of sRP and the morbidity associated with this operation [11]. As such, when an initial curative salvage option is considered, repeat radiation and ablation techniques-including, cryotherapy and high-intensity focused ultrasound-are being employed more frequently [1213].
Patients presenting with locally recurrent cancer after multiple local treatments can be a technical challenge for performing sRP. The surgical experience presented in this small cohort of patients reinforces the feasibility of performing the sRP even following multiple local treatments but the potential for morbidity is high. Prior to sRP, some patients have already experienced significant treatmentrelated complication including urethral stricture, urinary incontinence, and rectourethral fistula. Additional surgical procedures can be performed during sRP to address these pre-existing problems. As mentioned earlier, the surgery is technically challenging and potential intra- and post-operative complications must be discussed with the patient prior to sRP. Although the majority of our experience in this group of patients were performed as an open procedure and we were able adapt minimally invasive approach in the latter part of this series.
A significant complication after sRP is an anastomotic stricture which typically requires multiple surgical procedures to manage and invariably results in complete incontinence. Indeed, of the 4 men with an anastomotic stricture in our series two ultimately required urinary diversion to manage this complication. Previous reports have noted that the risk of anastomotic stricture in significantly lower after robotic assisted than open radical prostatectomy in both the primary and salvage setting [14]. The two patients treated with robotic surgery in our series did not develop an anastomotic stricture. While this by no means proves the point the expectation is that robotic-assistance in this setting will markedly reduce this complication.
Post operative complications were common and additional procedures and longer hospital stay is the result. The common complications noted in our series were anastomotic leak, anastomotic stricture and stress urinary incontinence. Artificial urinary sphincter, cystectomy with urinary diversion and perineal urethroplasty were the major surgical procedures performed after sRP. Nevertheless, all the patients were off ADT post-operatively, 91% of the patients achieved undetectable PSA in the post-operative period and 70% continue disease-free at last follow-up. Among the 3 patients having BCR, 2 were started on ADT and 1 patient is being actively monitored.
Chade et al. [15] performed systematic review outcomes of sRP and demonstrated 5 years BCR free probability varied between 47% and 82% and cancer specific survival at 10 years was 83%. Vast majority of patients in this review are most likely received sRP after initial recurrence after RT without any additional local therapies. Major complications (Clavien 3–5) were noted in up to 25% and EBL was 119–1,000 mL. Continence after sRP ranged between 21% to 90% and erectile dysfunction was as high as 90%. At this stage, it is difficult to accurately define if the surgical complexity increases proportionally with the number of local treatments or if sRP following any particularly type of ablation is better than the other therapy. It is highly plausible that more local treatment is likely to induce severe peri-prostatic tissue destruction which will contribute to the surgical complexity, complications and morbidity.
The limitations of this study are the small cohort size and retrospective series without a comparison arm. The follow-up in the current series is relatively short after sRP, however considering these patients are very-high risk populations, failures are more likely to occur soon after local therapy. The advantage of using robotic platform in this cohort of complex operations needs to be further explored [1617]. Overall, it is important to reemphasize the complexity of sRP following multiple local treatments; while cancer control appears excellent this must be balanced by concerns of surgical morbidity.
sRP for recurrent prostate cancer following multiple local treatments is challenging but provides excellent control of cancer. Patients often presented with complications from previous local treatments and additional surgical procedures can be electively planned along with sRP to correct such complications. Higher rates of post-operative complications were observed and may require additional surgical procedures to address the complications.
References
1. Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010; 28:1117–1123. PMID: 20124165.

2. Zelefsky MJ, Ben-Porat L, Scher HI, Chan HM, Fearn PA, Fuks ZY, et al. Outcome predictors for the increasing PSA state after definitive external-beam radiotherapy for prostate cancer. J Clin Oncol. 2005; 23:826–831. PMID: 15681527.

3. Shipley WU, Thames HD, Sandler HM, Hanks GE, Zietman AL, Perez CA, et al. Radiation therapy for clinically localized prostate cancer: a multi-institutional pooled analysis. JAMA. 1999; 281:1598–1604. PMID: 10235152.

4. Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff RO, Storme G, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010; 11:1066–1073. PMID: 20933466.

5. Zagars GK, Pollack A. Kinetics of serum prostate-specific antigen after external beam radiation for clinically localized prostate cancer. Radiother Oncol. 1997; 44:213–221. PMID: 9380819.

6. Agarwal PK, Sadetsky N, Konety BR, Resnick MI, Carroll PR. Cancer of the Prostate Strategic Urological Research Endeavor (CaPSURE). Treatment failure after primary and salvage therapy for prostate cancer: likelihood, patterns of care, and outcomes. Cancer. 2008; 112:307–314. PMID: 18050294.
7. Tetreault-Laflamme A, Crook J. Options for salvage of radiation failures for prostate cancer. Semin Radiat Oncol. 2017; 27:67–78. PMID: 27986213.

8. Eastham JA, DiBlasio CJ, Scardino PT. Salvage radical prostatectomy for recurrence of prostate cancer after radiation therapy. Curr Urol Rep. 2003; 4:211–215. PMID: 12756084.

9. Matsushita K, Ginsburg L, Mian BM, De E, Chughtai BI, Bernstein M, et al. Pubovesical fistula: a rare complication after treatment of prostate cancer. Urology. 2012; 80:446–451. PMID: 22698471.

10. Lee WR, Hanks GE, Hanlon A. Increasing prostate-specific antigen profile following definitive radiation therapy for localized prostate cancer: clinical observations. J Clin Oncol. 1997; 15:230–238. PMID: 8996147.

11. Stephenson AJ, Scardino PT, Bianco FJ Jr, DiBlasio CJ, Fearn PA, Eastham JA. Morbidity and functional outcomes of salvage radical prostatectomy for locally recurrent prostate cancer after radiation therapy. J Urol. 2004; 172:2239–2243. PMID: 15538239.

12. Pisters LL, Rewcastle JC, Donnelly BJ, Lugnani FM, Katz AE, Jones JS. Salvage prostate cryoablation: initial results from the cryo on-line data registry. J Urol. 2008; 180:559–563. discussion 563-4. PMID: 18554664.

13. Zacharakis E, Ahmed HU, Ishaq A, Scott R, Illing R, Freeman A, et al. The feasibility and safety of high-intensity focused ultrasound as salvage therapy for recurrent prostate cancer following external beam radiotherapy. BJU Int. 2008; 102:786–792. PMID: 18564135.

14. Kaffenberger SD, Smith JA. Salvage robotic radical prostatectomy. Indian J Urol. 2014; 30:429–433. PMID: 25378826.

15. Chade DC, Eastham J, Graefen M, Hu JC, Karnes RJ, Klotz L, et al. Cancer control and functional outcomes of salvage radical prostatectomy for radiation-recurrent prostate cancer: a systematic review of the literature. Eur Urol. 2012; 61:961–971. PMID: 22280856.

16. Orré M, Piéchaud T, Sargos P, Richaud P, Roubaud G, Thomas L. Oncological and functional results of robotic salvage radical prostatectomy after permanent brachytherapy implants. Cancer Radiother. 2017; 21:119–123. PMID: 28396223.

17. Ou YC, Hung SC, Hwang LH, Yang CK, Hung SW, Tung MC. Salvage robotic-assisted laparoscopic radical prostatectomy: experience with 14 cases. Anticancer Res. 2017; 37:2045–2050. PMID: 28373480.
Table 1
Clinical characteristics prior to salvage radical prostatectomy (n=11)
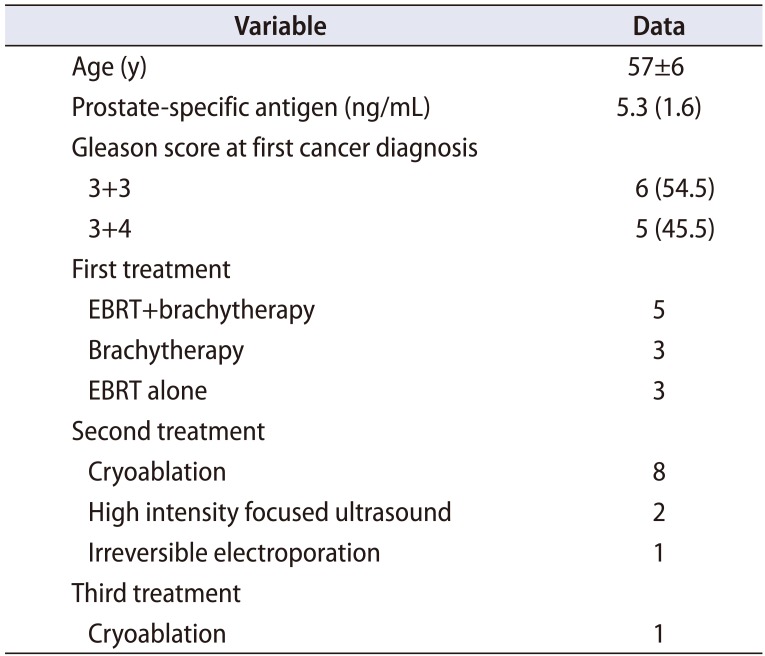
Table 2
Clinical characteristics at salvage radical prostatectomy (n=11)
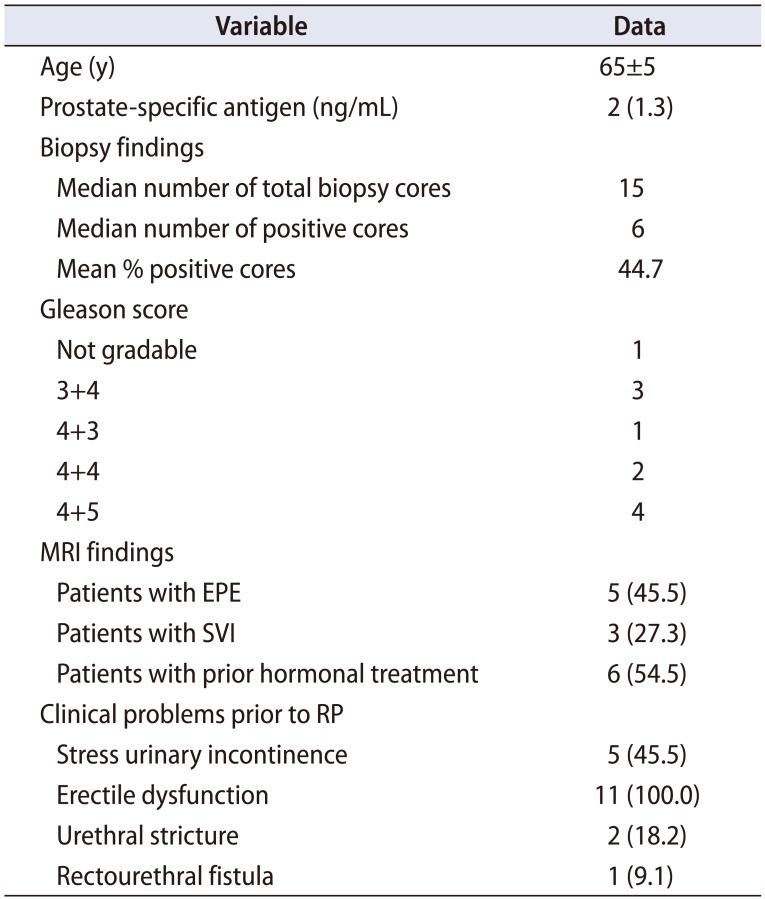
Table 3
Brief summary of each case detail
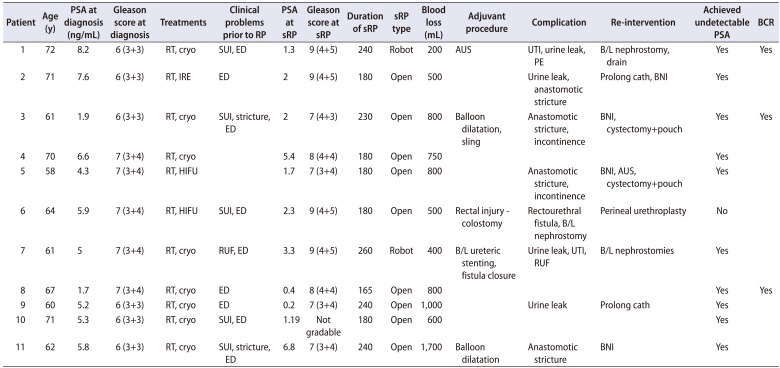
PSA, prostate-specific antigen; RP, radical prostatectomy; sRP, salvage RP; BCR, biochemical recurrence; RT, radiotherapy; cryo, cryotherapy; SUI, stress urinary incontinence; ED, erectile dysfunction; AUS, artificial urinary sphincter; UTI, urinary tract infection; PE, pulmonary embolism; B/L, bilateral; IRE, irreversible electroporation; BNI, bladder neck incisions; HIFU, high intensity focused ultrasound; RUF, recto-urethral fistula.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download