Abstract
Purpose
To evaluate the rate of pyuria and bacteriuria after transurethral resection of bladder tumor (TURBT).
Materials and Methods
We retrospectively evaluated data obtained from 363 patients who underwent TURBT between October 2012 and December 2013 at Seoul National University Hospital. Urinalysis and urine culture were assessed at 3, 6, 12, and 24 months postoperatively. Primary endpoint was the rate of bacteriuria (≥105/mL in a midstream) and pyuria (white blood cells ≥5/high-power field).
Results
We analyzed 306 patients who were eligible for the study. Pyuria was present in 23.5% of patients in the 3rd postoperative month and in 31.7% of patients in the 24th postoperative month. Bacteriuria was present in 1.3% of patients in the 3rd postoperative month and in 2.6% of patients in the 24th postoperative month. Among urothelial carcinoma patients (n=220), 24.1% showed pyuria and 1.8% showed bacteriuria at the 3rd postoperative month. We found that 31.8% showed pyuria and 3.2% showed bacteriuria at the 24th postoperative month. There was no significant difference in the rate of pyuria and bacteriuria between the intravesical treatment group and the no-treatment group. Multivariate analysis demonstrated that pyuria in the 3rd postoperative month (odd ratio [OR], 2.254; p=0.039), tumor multiplicity (OR, 3.331; p=0.001), and the absence of intravesical treatment (OR, 4.927; p=0.001) increases the risk of tumor recurrence.
Urinary bladder cancer is one of the most common urologic malignancies [1]. Notwithstanding a lower incidence of bladder cancer in Korea compared to Western countries, it is noted to be the second common urological cancer among Koreans [2]. Transurethral resection of bladder tumor (TURBT) constitutes the first-line treatment and diagnostic modality for bladder cancer [3]. Intravesical chemotherapy or immunotherapy is routinely used worldwide as adjunctive management after TURBT to prevent or delay the recurrence of bladder cancer [4]. However, irritative symptoms are commonly found in patients who undergo TURBT with or without subsequent intravesical therapy [5]. These symptoms resemble those found in cases with an overactive bladder such as urgency, frequency, and urge incontinence [6]. Earlier irritative symptoms were found to negatively affect the patients' recovery, sequential therapy, and daily living. Zhang et al. [7] reported that solifenacin can be beneficial in the management of irritative symptoms after TURBT. However, previous studies did not include patients who showed pyuria or bacteriuria.
It is known that most cases of asymptomatic bacteriuria (ASBU) do not require treatment. However, based on European Association of Urology (EAU) guidelines, urological procedures necessitate pre-procedure screening for bacteriuria due to the procedure-associated risk of mucosal bleeding that predisposes patients to the risk of post-procedure bacteremia and sepsis. Treatment of ASBU in these situations has been demonstrated to prevent these complications. EAU Guidelines recommend that regular cystoscopic evaluation and evaluation of urinary biomarkers be performed in patients during follow-up after a diagnosis of bladder cancer. Cystoscopy could cause potential mucosal tearing of the urothelium or prostate. To date, no study has evaluated the incidence of pyuria or bacteriuria after TURBT. Thus, we investigated the incidence rate of pyuria or bacteriuria after TURBT and aimed to assess the clinical relevance of persistent lower urinary tract infection (UTI) after surgery.
The Institutional Review Board of Seoul National University Hospital approved this study (approval number: H-1707-070-869). Because ours was a retrospective study, the IRB waived the need to obtain informed consent from our patients. Patient information was anonymized and de-identified before we performed the study. All study procedures were performed based on the Declaration of Helsinki guidelines.
We retrospectively evaluated data obtained from 363 patients who underwent TURBT between October 2012 and December 2013 at Seoul National University Hospital. Medical records were reviewed for tumor category, presence of comorbidities, urinalysis, age at the time of operation, and gender. Patients with visible tumors underwent complete transurethral bladder resection and were staged based on the 1987 TNM classification and the World Health Organization 1973 grading system. One gram of flomoxef sodium was administered once before and once after surgery. No additional antibiotics were administered at the time of discharge. If the pre-operative urinalysis confirmed the presence of bacteria, sensitive antibiotics were used. Patients were followed-up in an outpatient setting based on contemporary guidelines (EAU Guidelines). Urine analysis and urine culture were evaluated at 3, 6, 12, and 24 months postoperatively.
We excluded patients who were diagnosed with muscleinvasive urothelial carcinoma after TURBT, those with a previous history of upper tract urothelial carcinoma (UTUC), those showing pathological stage T3 or T4, and those >89 years of age. Primary endpoint was bacteriuria (≥105/mL in a midstream sample) and pyuria (white blood cells [WBCs] ≥5/high-power field [HPF]) detected using microscopic examination of urine] at 3, 6, 12, and 24 months postoperatively. Secondary endpoint was the rate patients of total symptomatic acute UTI (dysuria, frequency, burning pain at micturition) episode during 24 months. Additionally, we also determined the rate of antibiotic use for UTI during 24 months.
Logistic regression analysis and chi-square tests were performed to assess individual risk factors. Factors associated with dependent variables at a value of p<0.1 were included in the multivariate logistic regression model. We analyzed data using the IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA). Analytic statistics were obtained using the chi-square test or the Fisher exact test, and differences were considered statistically significant if the p-value was <0.05.
Among the consecutive 363 patients treated using TURBT during the study period, we excluded 10 patients >80 years, six patients who related history of previous pT3 or UTUC, and 32 patients with history of having undergone radical cystectomy within 6 months after the TURBT (23 patients diagnosed with muscle-invasive urothelial carcinoma after TURBT). Thus, 306 patients were eligible for inclusion in our study. Patient characteristics have been shown in Table 1. Among these patients, 220 patients were diagnosed with urothelial carcinoma, and among these 220, 71.8% patients were diagnosed with pathologic Ta, 7.3% of patients showed concomitant carcinoma in situ, while 53.6% patients were treated using intravesical treatment.
The rate of patients with bacteriuria and pyuria after TURBT is shown in Figs. 1, 2. Dipstick pyuria was present in 23.5% of patients at the 3rd postoperative month and in 31.7% at the 24th postoperative month. Bacteriuria was present in 1.3% of patients at the 3rd postoperative month and 2.6% of patients at the 24th postoperative month. Among the patients diagnosed with urothelial carcinoma, 24.1% showed pyuria and 1.8% showed bacteriuria in the 3rd postoperative month (Figs. 1, 2). Moreover, 31.8% of patients showed pyuria and 3.2% showed bacteriuria at the 24th postoperative month. Antibiotic treatment was administered to 9.5% of patients and 4.0% of patients showed persistent UTI symptoms following antibiotic treatment during follow-up. Among patients diagnosed with urothelial carcinoma, 10.0% of patients received additional antibiotic treatment and 4.5% showed persistent UTI symptoms (Table 1).
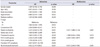
Among patients diagnosed with urothelial carcinoma, 118 patients received intravesical treatment during the follow-up period (Table 2). Among them, 89 patients were treated with bacillus Calmette-Guérin (BCG) and 29 patients were treated with mitomycin C (MMC). Intravesical treatments were repeated each week for 6 weeks. All patients did not receive routine antibiotics after intravesical treatment. There was no significant difference in the rate of pyuria and bacteriuria between the intravesical treatment group and no-treatment group (p>0.05).
Tumor recurrence was observed in 98 patients (44.5%). A significant difference in the rate of pyuria was observed between the tumor recurrence group and no-recurrence group at the 3rd postoperative month (p=0.006) and at the 6th postoperative month (p=0.042) (Table 3). Additionally, there was also difference rate of patients had persistent UTI symptoms between tumor recurrence group with no-recurrence group (p=0.044). Results of univariate and multivariate tests are shown in Table 4. Univariate analysis revealed that pyuria in the 3rd postoperative month, preoperative pyuria, tumor multiplicity, and the absence of intravesical treatment were significant predictors for tumor recurrence (p<0.05). Univariate analysis showed that pyuria at the 6th postoperative month and persistent UTI symptoms were not significant predictors but showed a significant tendency. Variables included in the final multivariate model for recurrence were pyuria in the 3rd postoperative month, preoperative pyuria, tumor multiplicity, and no intravesical treatment. Multivariate analysis demonstrated that pyuria in the 3rd postoperative month (odd ratio [OR], 2.254; p=0.039), tumor multiplicity (OR, 3.331; p=0.001), and absence of intravesical treatment (OR, 4.927; p=0.001) were significantly correlated with an increased risk of tumor recurrence.
ASBU is a condition in which patients demonstrating positive urine cultures do not show signs or symptoms of a UTI. Similarly, asymptomatic pyuria (ASPU) is the presence of pyuria in patients who do not show signs or symptoms of a UTI. Clinical studies have shown that ASBU and ASPU may protect against superinfecting symptomatic UTI; thus, treatment of ASBU and ASPU is warranted only in cases of proven benefit to the patient to avoid the risk of precipitating antimicrobial resistance and eradicating a potentially protective ASBU strain [89]. Screening and treatment for ASBU or ASPU should be administered in patients only if the bacteriuria is accompanied by adverse outcomes such as development of a symptomatic UTI, and bacteremia among others, which can be prevented by using antimicrobial therapy. However, most cases of ASBU or ASPU do not require treatment [10]. Regardless of these recommendations, injudicious and overuse of antibiotics is common in patients who are treated for ASBU and ASPU.
The use of antibiotic prophylaxis in patients undergoing diagnostic cystoscopy in outpatient settings is controversial. Although the EAU guidelines recommend their use only in high-risk patients, antibiotic use in routine clinical practice largely depends on the preferences of the treating urologist [11]. Some clinicians use prophylactic antibiotics demonstrated that cystoscopy can injury mucosal of urinary tract and can cause lower UTI. However, in their prospective study, Jiménez-Pacheco et al. [12] and Arrabal-Polo et al. [13] did not observe any benefit with antibiotic prophylaxis, in a population wherein risk factors were not appropriately selected. Based on recent studies investigating a very large cohort, Herr [14] has shown that antibiotic therapy administered prior to cystoscopy does not appear to be necessary in patients without signs or symptoms of a UTI. We prescribe antibiotics only to patients who warrant use of these medications as dictated by their clinical presentation. Based on the policies followed at our hospital, we do not routinely prescribe antibiotics after cystoscopy or intravesical treatment in an outpatient setting.
Ours is the first study to describe the rate of lower UTI after TURBT, although previous studies have attempted to determine the significance of pyuria in patients diagnosed with bladder cancer. Azuma et al. [15] have reported that patients with inflammatory non-muscle-invasive bladder cancer (NMIBC) show poor clinical outcomes. Authors reported in their study that 24% of patients demonstrated pyuria (a finding similar to our study results, as defined by urine showing WBCs ≥10/HPF. However, unlike in our study, that study employed very strict criteria for assessment of pyuria, and rate of bacteriuria was not determined. Satake et al. [16] demonstrated through their retrospective study that the presence of preoperative pyuria seemed to be significantly associated with recurrence in patients diagnosed with NMIBC. Their study showed that 116 of 237 patients (48.9%) had preoperative pyuria. In our study, 116 of 220 patients (52.7%) had preoperative pyuria. However, their study differs from ours in that it did not reflect the rate of bacteriuria and postoperative pyuria.
In this study, presence of pyuria at the 3rd postoperative month and no intravesical treatment were identified as independent risk factors to predict disease recurrence. It is known that intravesical treatment reduces recurrence of bladder tumors, delays progression, and improves overall survival [17]. However, few studies have evaluated the role of pyuria as a risk factor predicting recurrence during short-term follow-up after TURBT in patients diagnosed with urothelial carcinoma. Similarly, Satake et al. [16], reported in their retrospective analysis that preoperative pyuria was a significant risk factor for NMIBC. The mechanism underlying the association between pyuria and bladder cancer recurrence has not yet been elucidated. Many authors have reported that leukocytes could play a key role in cancer-associated inflammation [1819]. Although the significance of leukocytes in the urine of patients with NMIBC has not been clearly explained based on previous studies, our study shows that postoperative pyuria detected during short-term follow-up could be a potential risk factor to predict urothelial carcinoma. In addition, our study is useful because it demonstrates that a simple urine test (to detect the presence of pyuria) could be valuable in predicting risk of tumor recurrence during follow-up in cancer patients. In our study, presence of preoperative pyuria did not correlate with recurrence, and we did not investigate survival rates in patients diagnosed with bladder cancer. We propose that further studies involving larger study populations would be required to elucidate the nature of this association.
In this study, we found no difference between the intravesical treatment group and the no-treatment group with respect to the rate of pyuria and bacteriuria. Poletajew et al. [20] have reported in their systematic review that ASBU does not negatively affect the safety and efficacy of intravesical BCG immunotherapy. The authors suggested that there is no evidence to support routine screening for and treatment of ASBU in patients scheduled for intravesical BCG instillations in those diagnosed with bladder cancer. We do not routinely administer antibiotics after intravesical BCG instillation at our hospital. We reckon that frequent catheterization may evoke pyuria. Contrary to our assumption, except for the first 3 months, we found that the difference in the ratio of pyuria to bacteriuria was unclear in this study.
The significant rate of pyuria demonstrated after TURBT could be attributed to the following mechanisms: 1) The process of wound healing can affect development of postoperative ASPU. Some studies report that the healing process in the bladder involves inflammation, proliferation, and remodeling including production of extracellular matrix and increased quantities of growth factors [212223]. We hypothesize that this mechanism of bladder healing can affect the presence of persistent pyuria or bacteriuria after TURBT. However, these mechanisms are possibly related to urinary infections in the short term after surgery, and might not be applicable in the long term. 2) Disruption of urothelium and altered urothelial physiology may interfere with sterilization of urine. The urothelium protects underlying bladder tissues, and in general our body, against urine natural constituents, irritants, toxins, and infections. Adherence of uropathogenic Escherichia coli to uroplakin proteins on the apical surface of umbrella cells causes UTIs [2425]. Adherence is followed by bacterial invasion of the injured bladder wall [26]. Schilling and Hultgren [25] have reported that internalization of uropathogenic E. coli in the umbrella cells and formation of intracellular colonies (biofilm-like pods) of uropathogenic E. coli in umbrella cells are mechanisms that play a key role in the development of chronic UTI. Therefore, we hypothesize that pyuria following TURBT can be attributed to the above-mentioned causative mechanisms. In our study, the prevalence of pyuria increased with the passage of time. It increased or decreased notably, but decreased significantly compared with the preoperative ratio. Persistent pyuria after surgery seems to have been influenced by additional interventions after surgery.
Limitations to our study include: 1) Ours was a retrospective study. A cohort involving a larger sample size, designed to provide long-term follow-up could effectively evaluate the correlation between postoperative pyuria (bacteriuria) and the progression or recurrence-free survival of bladder cancer. 2) As mentioned earlier, we did not include preoperative urine analysis in this study. However, bladder cancer itself can present with pyuria and hematuria due to the fact that bladder cancer can cause pyuria. Therefore, we did not include the assessment of preoperative pyuria in this analysis, but intend to include this in subsequent studies.
Patients who have undergone TURBT may show a high rate of pyuria, although we noted no significant difference between the intravesical treatment group and the no-intravesical treatment group in this regard. Using univariate and multivariate analysis, detection of pyuria during the course of short-term follow-up was revealed to be a significant risk factor associated with the recurrence of urothelial carcinoma. A cohort study involving a larger sample size would be required to determine the correlation between postoperative pyuria (bacteriuria) and the progression or recurrence-free survival of bladder cancer. Such a study could provide more accurate results.
Figures and Tables
Fig. 1
The rate of pyuria over time after transurethral resection of bladder tumor (all patients, n=306; urothelial cancer, n=220). POD, postoperative day.
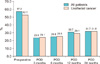
Fig. 2
The rate of bacteriuria over time after transurethral bladder tumor resection (all patients, n=306; urothelial cancer, n=220). POD, postoperative day.

Table 1
Baseline characteristics of patients who have undergone transurethral resection of bladder tumors (n=306)
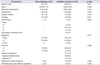
Table 2
Subgroup analysis performed on urothelial carcinoma patients comparing between the intravesical treatment and no-treatment group

Table 3
Subgroup analysis performed in urothelial carcinoma patients comparing between recurrence and no-recurrence group

Table 4
Univariate and multivariate analyses of risk factors for recurrence
References
1. Haresh KP, Julka PK, Sharma DN, Rath GK, Prabhakar R, Seth A. A prospective study evaluating surgery and chemo radiation in muscle invasive bladder cancer. J Cancer Res Ther. 2007; 3:81–85.

2. Joung JY, Lim J, Oh CM, Jung KW, Cho H, Kim SH, et al. Current trends in the incidence and survival rate of urological cancers in Korea. Cancer Res Treat. 2017; 49:607–615.

3. Divrik RT, Sahin AF, Yildirim U, Altok M, Zorlu F. Impact of routine second transurethral resection on the long-term outcome of patients with newly diagnosed pT1 urothelial carcinoma with respect to recurrence, progression rate, and disease-specific survival: a prospective randomised clinical trial. Eur Urol. 2010; 58:185–190.

4. Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007; 178:2314–2330.

5. Thrasher JB, Crawford ED. Complications of intravesical chemotherapy. Urol Clin North Am. 1992; 19:529–539.

6. Abrams P, Andersson KE, Birder L, Brubaker L, Cardozo L, Chapple C, et al. Fourth International Consultation on Incontinence Recommendations of the International Scientific Committee: evaluation and treatment of urinary incontinence, pelvic organ prolapse, and fecal incontinence. Neurourol Urodyn. 2010; 29:213–240.

7. Zhang Z, Cao Z, Xu C, Wang H, Zhang C, Pan A, et al. Solifenacin is able to improve the irritative symptoms after transurethral resection of bladder tumors. Urology. 2014; 84:117–121.

8. Hansson S, Jodal U, Lincoln K, Svanborg-Edén C. Untreated asymptomatic bacteriuria in girls: II--effect of phenoxymethylpenicillin and erythromycin given for intercurrent infections. BMJ. 1989; 298:856–859.

9. Cai T, Mazzoli S, Mondaini N, Meacci F, Nesi G, D'Elia C, et al. The role of asymptomatic bacteriuria in young women with recurrent urinary tract infections: to treat or not to treat? Clin Infect Dis. 2012; 55:771–777.

10. Raz R. Asymptomatic bacteriuria. Clinical significance and management. Int J Antimicrob Agents. 2003; 22:Suppl 2. 45–47.

11. Cai T, Verze P, Brugnolli A, Tiscione D, Luciani LG, Eccher C, et al. Adherence to European association of urology guidelines on prophylactic antibiotics: an important step in antimicrobial stewardship. Eur Urol. 2016; 69:276–283.

12. Jiménez-Pacheco A, Lardelli Claret P, López Luque A, Lahoz-García C, Arrabal Polo MA, Nogueras Ocaña M. Randomized clinical trial on antimicrobial prophylaxis for flexible urethrocystoscopy. Arch Esp Urol. 2012; 65:542–549.
13. Arrabal-Polo MA, Cano-García MDC, Arrabal-Martín M, Merino-Salas S. The effect of antibiotic prophylaxis on post-operative infection in patients undergone flexible cystos-copy. Urol J. 2017; 14:3050–3053.
14. Herr HW. Should antibiotics be given prior to outpatient cystoscopy? A plea to urologists to practice antibiotic stewardship. Eur Urol. 2014; 65:839–842.

15. Azuma T, Nagase Y, Oshi M. Pyuria predicts poor prognosis in patients with non-muscle-invasive bladder cancer. Clin Genitourin Cancer. 2013; 11:331–336.

16. Satake N, Ohno Y, Nakashima J, Ohori M, Tachibana M. Prognostic value of preoperative pyuria in patients with non-muscle-invasive bladder cancer. Int J Urol. 2015; 22:645–649.

17. Babjuk M, Böhle A, Burger M, Capoun O, Cohen D, Compérat EM, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol. 2017; 71:447–461.

18. Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest. 2010; 120:1151–1164.
19. Liang S, Sharma A, Peng HH, Robertson G, Dong C. Targeting mutant (V600E) B-Raf in melanoma interrupts immunoediting of leukocyte functions and melanoma extravasation. Cancer Res. 2007; 67:5814–5820.

20. Poletajew S, Zapała P, Radziszewski P. Safety and efficacy of intravesical Bacillus Calmette-Guérin immunotherapy in patients with non-muscle-invasive bladder cancer presenting with asymptomatic bacteriuria: a systematic review. Urol Int. 2017; 99:1–5.

21. Bilbao M, Spaniol A, Bearss J, Schellhase C, Shippey S, Aungst M. Histology surrounding cystotomy healing in a Sprague-Dawley rat model. Int Urogynecol J. 2014; 25:97–101.

22. Baskin LS, Sutherland RS, Thomson AA, Nguyen HT, Morgan DM, Hayward SW, et al. Growth factors in bladder wound healing. J Urol. 1997; 157:2388–2395.

23. Wishnow KI, Johnson DE, Grignon DJ, Cromeens DM, Ayala AG. Regeneration of the canine urinary bladder mucosa after complete surgical denudation. J Urol. 1989; 141:1476–1479.

24. Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003; 301:105–107.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download