Abstract
Underactive bladder (UAB), which has been described as a symptom complex suggestive of detrusor underactivity, is usually characterized by prolonged urination time with or without a sensation of incomplete bladder emptying, usually with hesitancy, reduced sensation on filling, and slow stream often with storage symptoms. Several causes such as aging, bladder outlet obstruction, diabetes mellitus, neurologic disorders, and nervous injury to the spinal cord, cauda equine, and peripheral pelvic nerve have been assumed to be responsible for the development of UAB. Several contributing factors have been suggested in the pathophysiology of UAB, including myogenic failure, efferent and/or afferent dysfunctions, and central nervous system dysfunction. In this review article, we have described relationships between individual contributing factors and the pathophysiology of UAB based on previous reports. However, many pathophysiological uncertainties still remain, which require more investigations using appropriate animal models.
Underactive bladder (UAB) has yet to be formally defined by any international academic society, such as the International Continence Society (ICS). Recently, an international expert group has described UAB as a symptom complex suggestive of detrusor underactivity (DU) that is usually characterized by prolonged urination time with or without a sensation of incomplete bladder emptying, usually with hesitancy, reduced sensation on filling, and slow stream [1]. Moreover, given Andersson's proposal to characterize UAB based on symptoms, a better term may be UAB syndrome, in analogy to the overactive bladder syndrome defined by the ICS [2]. Unlike UAB, DU has already been defined by the ICS as a contraction of reduced strength and/or duration resulting in prolonged or incomplete emptying of the bladder [3]. DU, which is urodynamically diagnosed based on a pressure-flow study, is characterized by a low-pressure, poorly sustained, or wavelike detrusor contraction associated with poor flow rate [4]. Considering its definition, UAB may have certain vagueness, given that the symptoms to be included in the syndrome still need to be discussed.
The lower urinary tract (LUT), including the urinary bladder and urethral sphincter, is regulated by simple circuits (on-off switching) that maintain an interactive relationship between the urinary bladder and the urethral outlet through which sufficient storage and voiding can be achieved. These reflexes are coordinated through complex neural interactions among the central nervous system (CNS) including pons, periaqueductal gray, brain frontal cortex, lumbosacral spinal cord, and peripheral nerves system (pelvic, hypogastric, and pudendal nerves), and storage and voiding reflexes are primarily organized in the spinal cord and the brain, respectively [5]. Sufficient urine output requires complete relaxation of the internal (smooth muscle) and external urethral sphincter (striated muscle), followed by increased intravesical pressure due to detrusor smooth muscle contraction [6]. It is highly probable that UAB is caused by one or several dysfunction(s) in the site (central and/or peripheral) innervating the micturition reflex.
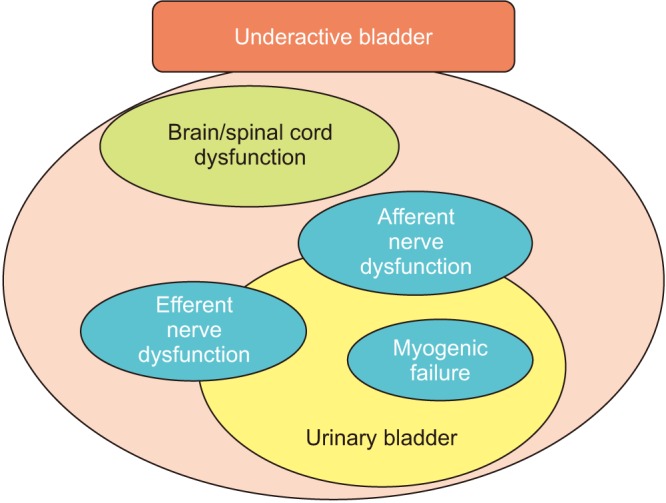
So far, several causes (diseases/disorders) of UAB have been reported [78]: aging [910], persistent bladder outlet obstruction (BOO) [11], diabetes mellitus (DM) [1213], neurologic disorders [14] (e.g., Parkinson disease, multiple sclerosis, and infectious neurologic problems, such as AIDS and herpes zoster), and nervous injury to the spinal cord, cauda equine, and peripheral nerves [1516] (e.g., spinal stenosis, pelvic fractures, and pelvic surgery). These cause impaired bladder function, especially voiding, and consequently result in DU/UAB, which undoubtedly deteriorates the patient's quality of life. To optimize treatment, we need to consider how such causes (diseases) contribute to impaired voiding function. Considering pathophysiological backgrounds, several contributing factors, such as intrinsic detrusor dysfunction (myogenic failure), efferent and/or afferent nerve dysfunctions, and dysfunctions in CNS control, have been reported (Fig. 1).
Human detrusor smooth muscle bundles are arranged in a complicated pattern and surrounded by connective tissue containing significant amounts of collagen. From a functional viewpoint, however, the detrusor expands an integrated unit of interconnected muscle bundles [17]. The different bundles converge in the bladder neck, continue to the urethra, and then run an oblique or longitudinal course in the urethral wall. The muscles fade out distally in the connective tissues surrounding the urethral meatus. Impairment in the contractility of the detrusor smooth muscle, which can be attributed to “myogenic failure,” would directly lead to DU/UAB.
One of the major causes of myogenic failure is “aging.” Symptoms of urinary retention, urinary hesitancy, and incontinence, which are frequently observed with aging, have been attributed to UAB [181920]. Gilpin et al. [21] reported on age-related morphological changes, especially decreased axonal content of the human detrusor smooth muscle. Another histological examination that utilized human tissues demonstrated an increase in collagen deposition with aging [22]. In patients with UAB, similar histological changes, such as widespread degeneration of the axon, muscle loss, and fibrosis of the detrusor smooth muscle, were observed [23]. Moreover, Mansfield et al. [24] reported that mRNA expression of M3 muscarinic receptor decreased with aging and these changes may diminish the potential sensitivity of micromotional activity to cholinergic neurotransmitters. These age-related changes were recently confirmed and reported by Ito et al. [2526]. They demonstrated that aged rats showed weaker contractile responses to carbachol and electrical field stimulation related to decreased cholinergic-mediated contraction, lower M3 muscarinic receptor mRNA expression, and higher collagen deposition in isolated detrusor strips, and that cystometric investigations of aged rats showed greater postvoid residual volume and lower voiding efficiency. Pathophysiological changes in muscarinic receptors, especially the M3 muscarinic receptor subtype, and fibrosis can be theoretically and reliably considered as key factors for UAB.
Voluntary control of the LUT requires nervous interactions between autonomic (sympathetic and parasympathetic nerves) and somatic (pudendal nerves) afferent and efferent pathways [5]. In several pathophysiological conditions of LUT dysfunction [2728293031], deterioration of bladder efferent function may be due to peripheral denervation. Various factors, such as nerve damage (e.g., DM) [3233], ischemic/oxidative conditions (e.g., BOO and atherosclerosis) [343536373839], and oxidative stress [40], have been assumed to induce peripheral denervation.
Several studies have indicated that the isolated bladder itself shows autonomous micromotions (microcontractions), which increase with bladder distension, generate sensory (afferent) nerve activity, and become altered in cases of LUT dysfunctions [41424344]. During the voiding phase in a normal bladder, excitation of the parasympathetic efferent nerve causes the smooth muscles to contract synchronously, thereby increasing intravesical pressure. However, individuals with bladder efferent function deterioration, especially by denervation, would have an attenuated initial contractile force in the denervated areas. Therefore, these denervated areas might be able to contract only when there is excited propagation from a neighboring intact innervated area or via interstitial cells that are also present between the bundles of detrusor smooth muscle. Moreover, during voiding, the potential for micromotional propagation to recruit contraction in denervated areas may allow compensation for some loss of innervation. However, over time, extensive denervation may take too long to generate a rise in tone and contraction, which would lead to prolonged voiding durations with insufficient urine evacuation. Thus, the increasing severity of denervation would be associated with an attenuation of contractibility, causing impaired detrusor contractility [45]. Such processes have been suggested at least in aged mice [46].
Throughout the bladder filling (storage) phase, the parasympathetic efferent innervation (pelvic nerve) to the detrusor is inhibited, whereas that to the urethral smooth and striated muscles (hypogastric and pudendal nerves) is activated, preventing urinary incontinence [47]. Bladder distention has been supposed to evoke afferent activity via myelinated Aδ-fibers connected in series with smooth muscle fibers [4849]. This in turn activates sympathetic outflow, (mainly via the hypogastric nerve), to the bladder outlet (the bladder neck and the urethra) and the pudendal outflow to the external urethral sphincter during the storage phase. Normal bladder distension activates low-threshold mechanoreceptive afferents coupled in series with detrusor muscle cells (myogenic pathway). However, unmyelinated C-fibers running in the suburothelial layer and even in the urothelium, coupled with urothelial cells and suburothelial interstitial cells, lead to signal transductions from the urothelium (urothelial pathway) [50]. Not only is the urothelium a barrier for harmful substances in the urine, it is also functionally active in the storage phase of micturition cycles [51]. In particular, the urothelium actively contributes to sensory functions, expressing various receptors for neurotransmitters [52], while urothelial cells are able to release neurotransmitters and signaling molecules, including nitric oxide, adenosine triphosphate, ACh, prostaglandins, substance P, and nerve growth factor [5354555657]. Urothelial cell-released substances may act directly on afferent nerves or indirectly via an action on suburothelial myofibroblasts (also referred to as “interstitial cells”) that lie near afferent nerves. Myofibroblasts are extensively linked to each other, as well as to afferent nerve fibers and detrusor smooth muscles, by gap junctions (electrical synapse) and can release substances that in turn act on afferent nerves [5]. Thus, urothelial cells and myofibroblasts are believed to contribute to sensory mechanisms in the urinary bladder by chemical coupling to the adjacent sensory nerves. Therefore, it is conceivable that age- or disease-related changes in the structure and function of the urothelium and afferent nerves could directly alter bladder afferent function.
Smith et al. [58] performed urodynamic studies in patients with LUT symptoms and found that those with DU may have defective volume sensation rather than impaired detrusor contractility, suggesting that reduced central sensitivity to volume sensation (i.e., mechanosensitive afferent activity) contributes to DU in nonobstructed, nonneurogenic symptomatic patients. Such observations are consistent with their previous reports wherein they show that peripheral and/or central sensory mechanisms may be important contributors to aging-related bladder dysfunction [959]. In addition, Azadzoi et al. [37] showed degenerating and collapsed axons, Schwann cells surrounded by dense connective tissue, and splitting of the myelin sheaths in a chronically ischemic bladder. Dahlin et al. [60] reported that thinly myelinated fibers were more susceptible to oxygen deprivation under ischemic conditions than thicker ones, whereas unmyelinated fibers were resistant to ischemic induction. Furthermore, attenuation of urothelial sensitivity to modulators of potassium channel activity has been observed in diabetic bladders [61], which may cause afferent sensory dysfunction [33626364], as well as contribute to contractile force and spontaneous activity of the detrusor smooth muscle [61]. Mohammed et al. [6566] reported that aged rats showed significant decreases in the expression of calcitonin gene-related peptide (CGRP) and substance P on the lumbosacral dorsal root ganglion neurons and in the density of pituitary adenylate cyclase-activating polypeptide innervation on the subepithelial plexus and the muscle layer of the bladder, whereas CGRP and substance P innervation on the muscle layer were slightly reduced. Moreover, Jiang and Kuo [67] recently reported that patients with DU had a significantly lower expression of E-cadherin, which plays an important role in cell adhesion. They additionally demonstrated a decrease in the expression of M2 and M3 muscarinic receptors, P2X3 purinergic receptors, and endothelial nitric oxide synthase and an increase in the expression of β3-adrenoceptors in patients with DU [67]. These findings clearly show that directly altered sensory transduction and impaired urothelial signaling pathways appear to be the pathophysiological conditions for UAB/DU.
Symptoms of incomplete bladder emptying, which has been attributed to UAB, are also commonly observed in patients with specific neurological diseases: multiple sclerosis [68], Parkinson disease [69], and multiple system atrophy [70]. The common pathologic findings in multiple sclerosis are focal demyelination and plaque formation throughout the CNS (brain to spinal cord), which delay and/or block nervous system communication (nervous conduction), including sensory afferent function [71]. Regarding the relationship between lesion sites and symptoms, a previous study demonstrated that patients with cervical cord or pontine lesions were more likely to suffer from UAB/DU, whereas those with cerebral cortex lesions were more prone to having storage symptoms, and detrusor overactivity (DO) [72]. Although the underlying pathophysiology of UAB/DU in Parkinson disease is still unclear, it is conceivable to correlate UAB/DU with the patient's overall motor function in the brain areas, including the frontal cortex, basal ganglia, thalamus, anterior cingulate gyrus, and caudate nucleus. Moreover, Kim et al. [73] recently reported that patients with multiple system atrophy had higher incidences of DU compared to those with Parkinson disease, although the pathophysiology of UAB/DU still remains uncertain.
Almost all patients with spinal cord injury (SCI) initially show UAB/DU or acontractile detrusor during spinal shock, which subsequently develop into chronic bladder dysfunctions depending on the level of spinal cord lesions. For patients with supra-lumbosacral SCI, the parasympathetic and sensory spinal centers in the sacral spinal cord are preserved; however, synaptic reorganization leads to the appearance of involuntary bladder contractions during bladder filling, i.e., neurogenic DO [747576]. In addition, the coordination between bladder contractions and urethral sphincter relaxation guaranteed by the pontine, where well known as micturition center, becomes impaired. Instead, bladder and urethral sphincter contractions occur simultaneously, such phenomenon leading to an event known as detrusor-sphincter dyssynergia.
Injuries in the sacral spinal cord or cauda equina could lead to chronically persisting DU or acontractile detrusor. In an animal model of cauda equina lesions, Sekido et al. [77] reported on the pathophysiology of UAB induced by lumbar spinal canal stenosis (LCS). Two weeks after surgery in rats with LCS, cystometry results showed that postvoid residual urine volume and the number of nonvoiding contractions increased, whereas voided volume, threshold pressure, and maximum intravesical pressure decreased. Moreover, isolated bladder strips showed an increase in the contractile response to electrical field stimulation. Interestingly, they reported no obvious changes in the detrusor muscle's contractile response to carbachol stimulation and its morphology in LCS rats.
Due to ethical concerns, direct experimentation on human subjects with UAB has been limited, requiring studies to utilize animal models of UAB as an alternative. Ideal animal models are those that mimic part of human pathophysiology and/or a functional problem. Thus far, several animal models mimicking clinical UAB, which show “prolonged urination time” and/or “reduced contractile strength,” have been reported, especially in rodents.
In an animal model related to DM, streptozocin-injected animals (rodents) have been widely used [323378]. This DM model is relatively guaranteed the experimental reproducibility with higher glucose blood level, but simultaneously needs to pay attention to the differentiation from the influence of polyuria, which can be distinguished by sucrose-induced polyuria animal model [63]. Moreover, genetical DM animals have long been used and recognized as mild DM model, which may be similar to type II DM in human [7980818283].
Surgery for the creation of BOO is basically similar among several studies, but the degree of obstruction sometimes depends on the techniques, animal species, and sexes [87]. Although a short period of time of obstruction shows sign of UAB [8889], prolonged periods of obstruction (chronic or persistent BOO) generally appears as more severe UAB [34899091].
There have been several published studies reported as a UAB model by using pelvic nerve crash- or cryoinjury (unilaterally or bilaterally) [9293949596], in which detrusor contractility was remarkably impaired. In case of bilateral pelvic nerve injury by complete resection, the animals showed severe nervous dysfunctions, which were resemble to the situation with SCI [95].
Ischemic models have long been investigated on bladder dysfunctions [60979899]. To address more clinical situation, atherosclerosis models have recently been reported [37100], and the animal with longer period of time of atherosclerosis may be a better candidate as an ideal UAB model [3839].
Given the absence of alternative tools for determining the pathophysiology of UAB, an animal model needs to be used. However, species differences between animals and humans should be considered. Moreover, an appropriate animal model of UAB has not been established, because UAB carries multifactorial symptoms and has yet to be properly def ined. To advance pathophysiological understanding and develop medical interventions including pharmacotherapy for UAB, various animal models need to be further established.
The pathophysiology of UAB/DU includes failure in detrusor muscle contractility, bladder efferent and afferent nerve dysfunction, and failure of the CNS to coordinate voiding function. Though a few publications have helped us better understand the complex pathophysiological mechanisms of UAB, many uncertainties still remain, particularly in the role of aging, altered sensory function, and the translational value of existing animal models.
Notes
CONFLICTS OF INTEREST: YI is a consultant for Astellas, Pfizer, and Eli Lilly; and has received grants from Astellas, Asahi Kasei, Kissei, Ono, Kyorin, Taiho, RaQualia, Daiichi-Sankyo, Nippon Shinyaku, Lilium Otsuka, Integral, Medicon, and Tsukada Medical Research; and speaker's fees from Astellas, Asahi Kasei, Kissei, Kyorin, Taiho, Nippon Shinyaku, and Pfizer.
References
1. Chapple CR, Osman NI, Birder L, van Koeveringe GA, Oelke M, Nitti VW, et al. The underactive bladder: a new clinical concept? Eur Urol. 2015; 68:351–353. PMID: 25770481.

3. Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002; 21:167–178. PMID: 11857671.

4. Cucchi A, Quaglini S, Rovereto B. Proposal for a urodynamic redefinition of detrusor underactivity. J Urol. 2009; 181:225–229. PMID: 19013594.

5. Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008; 9:453–466. PMID: 18490916.

6. de Groat WC. Integrative control of the lower urinary tract: preclinical perspective. Br J Pharmacol. 2006; 147(Suppl 2):S25–S40. PMID: 16465182.

7. Miyazato M, Yoshimura N, Chancellor MB. The other bladder syndrome: underactive bladder. Rev Urol. 2013; 15:11–22. PMID: 23671401.
8. Aggarwal H, Zimmern PE. Underactive bladder: current urology reports. Curr Urol Rep. 2016; 17:17. PMID: 26874529.
9. Smith PP. Aging and the underactive detrusor: a failure of activity or activation? Neurourol Urodyn. 2010; 29:408–412. PMID: 19760756.

10. Resnick NM, Yalla SV. Detrusor hyperactivity with impaired contractile function. An unrecognized but common cause of incontinence in elderly patients. JAMA. 1987; 257:3076–3081. PMID: 3586227.

11. Jiang YH, Lee CL, Kuo HC. Urothelial dysfunction, suburothelial inflammation and altered sensory protein expression in men with bladder outlet obstruction and various bladder dysfunctions: correlation with urodynamics. J Urol. 2016; 196:831–837. PMID: 26930253.

12. Frimodt-Moller C. Diabetic cystopathy: a review of the urodynamic and clinical features of neurogenic bladder dysfunction in diabetes mellitus. Dan Med Bull. 1978; 25:49–60. PMID: 344005.
13. Sasaki K, Yoshimura N, Chancellor MB. Implications of diabetes mellitus in urology. Urol Clin North Am. 2003; 30:1–12. PMID: 12580554.

14. Kadow BT, Tyagi P, Chermansky CJ. Neurogenic causes of detrusor underactivity. Curr Bladder Dysfunct Rep. 2015; 10:325–331. PMID: 26715948.

15. Chung DE, Dillon B, Kurta J, Maschino A, Cronin A, Sandhu JS. Detrusor underactivity is prevalent after radical prostatectomy: a urodynamic study including risk factors. Can Urol Assoc J. 2013; 7:E33–E37. PMID: 22277630.

16. Brooks RA, Wright JD, Powell MA, Rader JS, Gao F, Mutch DG, et al. Long-term assessment of bladder and bowel dysfunction after radical hysterectomy. Gynecol Oncol. 2009; 114:75–79. PMID: 19410279.

17. Gosling J. The structure of the bladder and urethra in relation to function. Urol Clin North Am. 1979; 6:31–38. PMID: 432999.

18. Elbadawi A, Hailemariam S, Yalla SV, Resnick NM. Structural basis of geriatric voiding dysfunction. VII. Prospective ultrastructural/urodynamic evaluation of its natural evolution. J Urol. 1997; 157:1814–1822. PMID: 9112530.

19. Resnick NM, Yalla SV, Laurino E. The pathophysiology of urinary incontinence among institutionalized elderly persons. N Engl J Med. 1989; 320:1–7. PMID: 2909873.

20. Taylor JA 3rd, Kuchel GA. Detrusor underactivity: clinical features and pathogenesis of an underdiagnosed geriatric condition. J Am Geriatr Soc. 2006; 54:1920–1932. PMID: 17198500.

21. Gilpin SA, Gilpin CJ, Dixon JS, Gosling JA, Kirby RS. The effect of age on the autonomic innervation of the urinary bladder. Br J Urol. 1986; 58:378–381. PMID: 3756405.

22. Lepor H, Sunaryadi I, Hartanto V, Shapiro E. Quantitative morphometry of the adult human bladder. J Urol. 1992; 148(2 Pt 1):414–417. PMID: 1378909.

23. Elbadawi A, Yalla SV, Resnick NM. Structural basis of geriatric voiding dysfunction. II. Aging detrusor: normal versus impaired contractility. J Urol. 1993; 150(5 Pt 2):1657–1667. PMID: 8411454.

24. Mansfield KJ, Liu L, Mitchelson FJ, Moore KH, Millard RJ, Burcher E. Muscarinic receptor subtypes in human bladder detrusor and mucosa, studied by radioligand binding and quantitative competitive RT-PCR: changes in ageing. Br J Pharmacol. 2005; 144:1089–1099. PMID: 15723094.

25. Ito H, Aizawa N, Fujita Y, Suzuki M, Fukuhara H, Homma Y, et al. Long-term caloric restriction in rats may prevent age related impairment of in vitro bladder function. J Urol. 2015; 193:2123–2130. PMID: 25451828.
26. Ito H, Kamei J, Aizawa N, Fujita Y, Suzuki M, Fukuhara H, et al. Preventive effects of long-term caloric restriction on aging related in vivo bladder dysfunction and molecular biological changes in the bladder and dorsal root ganglia in rats. J Urol. 2016; 196:1575–1583. PMID: 27259654.
27. Brading AF, Turner WH. The unstable bladder: towards a common mechanism. Br J Urol. 1994; 73:3–8. PMID: 8298896.

28. Mills IW, Greenland JE, McMurray G, McCoy R, Ho KM, Noble JG, et al. Studies of the pathophysiology of idiopathic detrusor instability: the physiological properties of the detrusor smooth muscle and its pattern of innervation. J Urol. 2000; 163:646–651. PMID: 10647702.

29. Sibley GN. The physiological response of the detrusor muscle to experimental bladder outflow obstruction in the pig. Br J Urol. 1987; 60:332–336. PMID: 3690205.

30. Drake MJ, Hedlund P, Mills IW, McCoy R, McMurray G, Gardner BP, et al. Structural and functional denervation of human detrusor after spinal cord injury. Lab Invest. 2000; 80:1491–1499. PMID: 11045565.

31. Drake MJ, Gardner BP, Brading AF. Innervation of the detrusor muscle bundle in neurogenic detrusor overactivity. BJU Int. 2003; 91:702–710. PMID: 12699489.

32. Daneshgari F, Liu G, Birder L, Hanna-Mitchell AT, Chacko S. Diabetic bladder dysfunction: current translational knowledge. J Urol. 2009; 182(6 Suppl):S18–S26. PMID: 19846137.

33. Christ GJ, Hsieh Y, Zhao W, Schenk G, Venkateswarlu K, Wang HZ, et al. Effects of streptozotocin-induced diabetes on bladder and erectile (dys)function in the same rat in vivo. BJU Int. 2006; 97:1076–1082. PMID: 16643495.

34. Schroder A, Chichester P, Kogan BA, Longhurst PA, Lieb J, Das AK, et al. Effect of chronic bladder outlet obstruction on blood flow of the rabbit bladder. J Urol. 2001; 165:640–646. PMID: 11176451.
35. Levin RM, Haugaard N, O'Connor L, Buttyan R, Das A, Dixon JS, et al. Obstructive response of human bladder to BPH vs. rabbit bladder response to partial outlet obstruction: a direct comparison. Neurourol Urodyn. 2000; 19:609–629. PMID: 11002303.

36. Levin RM, Schuler C, Leggett RE, Callaghan C, Maknuru S. Partial outlet obstruction in rabbits: duration versus severity. Int J Urol. 2013; 20:107–114. PMID: 23050656.

37. Azadzoi KM, Chen BG, Radisavljevic ZM, Siroky MB. Molecular reactions and ultrastructural damage in the chronically ischemic bladder. J Urol. 2011; 186:2115–2122. PMID: 21944111.

38. Nomiya M, Yamaguchi O, Akaihata H, Hata J, Sawada N, Kojima Y, et al. Progressive vascular damage may lead to bladder underactivity in rats. J Urol. 2014; 191:1462–1469. PMID: 24184364.

39. Zhao Z, Azad R, Yang JH, Siroky MB, Azadzoi KM. Progressive changes in detrusor function and micturition patterns with chroinc bladder ischemia. Investig Clin Urol. 2016; 57:249–259.

40. Malone L, Schuler C, Leggett RE, Levin RM. The effect of in vitro oxidative stress on the female rabbit bladder contractile response and antioxidant levels. ISRN Urol. 2013; 2013:639685. PMID: 23819065.

41. Drake MJ, Harvey IJ, Gillespie JI. Autonomous activity in the isolated guinea pig bladder. Exp Physiol. 2003; 88:19–30. PMID: 12525852.

42. Drake MJ, Harvey IJ, Gillespie JI, Van Duyl WA. Localized contractions in the normal human bladder and in urinary urgency. BJU Int. 2005; 95:1002–1005. PMID: 15839921.

43. Drake MJ, Hedlund P, Harvey IJ, Pandita RK, Andersson KE, Gillespie JI. Partial outlet obstruction enhances modular autonomous activity in the isolated rat bladder. J Urol. 2003; 170:276–279. PMID: 12796704.

44. Aizawa N, Homma Y, Igawa Y. Effects of mirabegron, a novel beta3-adrenoceptor agonist, on primary bladder afferent activity and bladder microcontractions in rats compared with the effects of oxybutynin. Eur Urol. 2012; 62:1165–1173. PMID: 22981677.
45. Drake MJ, Kanai A, Bijos DA, Ikeda Y, Zabbarova I, Vahabi B, et al. The potential role of unregulated autonomous bladder micromotions in urinary storage and voiding dysfunction; overactive bladder and detrusor underactivity. BJU Int. 2017; 119:22–29. PMID: 27444952.

46. Lagou M, Gillespie J, Kirkwood T, Harvey I, Drake MJ. Muscarinic stimulation of the mouse isolated whole bladder: physiological responses in young and ageing mice. Auton Autacoid Pharmacol. 2006; 26:253–260. PMID: 16879490.

47. de Groat WC, Yoshimura N. Afferent nerve regulation of bladder function in health and disease. Handb Exp Pharmacol. 2009; (194):91–138. PMID: 19655106.

48. Morrison J. The activation of bladder wall afferent nerves. Exp Physiol. 1999; 84:131–136. PMID: 10081713.

49. Iggo A. Tension receptors in the stomach and the urinary bladder. J Physiol. 1955; 128:593–607. PMID: 13243351.

50. Kanai A, Andersson KE. Bladder afferent signaling: recent findings. J Urol. 2010; 183:1288–1295. PMID: 20171668.

52. de Groat WC. The urothelium in overactive bladder: passive bystander or active participant? Urology. 2004; 64(6 Suppl 1):7–11. PMID: 15621221.

53. Birder LA, Apodaca G, De Groat WC, Kanai AJ. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol. 1998; 275(2 Pt 2):F226–F229. PMID: 9691011.
54. Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, et al. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002; 5:856–860. PMID: 12161756.

55. Birder LA, Nealen ML, Kiss S, de Groat WC, Caterina MJ, Wang E, et al. Beta-adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J Neurosci. 2002; 22:8063–8070. PMID: 12223560.
56. Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes: a possible sensory mechanism? J Physiol. 1997; 505(Pt 2):503–511. PMID: 9423189.
57. Yoshida M, Miyamae K, Iwashita H, Otani M, Inadome A. Management of detrusor dysfunction in the elderly: changes in acetylcholine and adenosine triphosphate release during aging. Urology. 2004; 63(3 Suppl 1):17–23.

58. Smith PP, Chalmers DJ, Feinn RS. Does defective volume sensation contribute to detrusor underactivity. Neurourol Urodyn. 2015; 34:752–756. PMID: 25220925.

59. Smith PP, DeAngelis A, Kuchel GA. Detrusor expulsive strength is preserved, but responsiveness to bladder filling and urinary sensitivity is diminished in the aging mouse. Am J Physiol Regul Integr Comp Physiol. 2012; 302:R577–R586. PMID: 22204955.

60. Dahlin LB, Shyu BC, Danielsen N, Andersson SA. Effects of nerve compression or ischaemia on conduction properties of myelinated and non-myelinated nerve fibres. An experimental study in the rabbit common peroneal nerve. Acta Physiol Scand. 1989; 136:97–105. PMID: 2773666.

61. Wang Y, Tar MT, Fu S, Melman A, Davies KP. Diabetes attenuates urothelial modulation of detrusor contractility and spontaneous activity. Int J Urol. 2014; 21:1059–1064. PMID: 24846346.

62. Kirschner-Hermanns R, Daneshgari F, Vahabi B, Birder L, Oelke M, Chacko S. Does diabetes mellitus-induced bladder remodeling affect lower urinary tract function? ICI-RS 2011. Neurourol Urodyn. 2012; 31:359–364. PMID: 22415965.

63. Xiao N, Wang Z, Huang Y, Daneshgari F, Liu G. Roles of polyuria and hyperglycemia in bladder dysfunction in diabetes. J Urol. 2013; 189:1130–1136. PMID: 22999997.

64. Aizawa N, Wakamatsu D, Kida J, Otsuki T, Saito Y, Matsuya H, et al. Inhibitory effects of retigabine, a Kv7 channel activator, on mechanosensitive primary bladder afferent activities and nociceptive behaviors in rats. Neurourol Urodyn. 2017; 36:280–285. PMID: 26536146.

65. Mohammed HA, Santer RM. Distribution and changes with age of calcitonin gene-related peptide- and substance P-immunoreactive nerves of the rat urinary bladder and lumbosacral sensory neurons. Eur J Morphol. 2002; 40:293–301. PMID: 15101445.

66. Mohammed H, Hannibal J, Fahrenkrug J, Santer R. Distribution and regional variation of pituitary adenylate cyclase activating polypeptide and other neuropeptides in the rat urinary bladder and ureter: effects of age. Urol Res. 2002; 30:248–255. PMID: 12202943.

67. Jiang YH, Kuo HC. Urothelial barrier deficits, suburothelial inflammation and altered sensory protein expression in detrusor underactivity. J Urol. 2017; 197:197–203. PMID: 27436428.

68. de Seze M, Ruffion A, Denys P, Joseph PA, Perrouin-Verbe B. GENULF. The neurogenic bladder in multiple sclerosis: review of the literature and proposal of management guidelines. Mult Scler. 2007; 13:915–928. PMID: 17881401.
69. Sakakibara R, Tateno F, Nagao T, Yamamoto T, Uchiyama T, Yamanishi T, et al. Bladder function of patients with Parkinson's disease. Int J Urol. 2014; 21:638–646. PMID: 24571321.

70. Bloch F, Pichon B, Bonnet AM, Pichon J, Vidailhet M, Roze E, et al. Urodynamic analysis in multiple system atrophy: characterisation of detrusor-sphincter dyssynergia. J Neurol. 2010; 257:1986–1991. PMID: 20683607.

71. Hinson JL, Boone TB. Urodynamics and multiple sclerosis. Urol Clin North Am. 1996; 23:475–481. PMID: 8701560.

72. Araki I, Matsui M, Ozawa K, Takeda M, Kuno S. Relationship of bladder dysfunction to lesion site in multiple sclerosis. J Urol. 2003; 169:1384–1387. PMID: 12629367.

73. Kim M, Jung JH, Park J, Son H, Jeong SJ, Oh SJ, et al. Impaired detrusor contractility is the pathognomonic urodynamic finding of multiple system atrophy compared to idiopathic Parkinson's disease. Parkinsonism Relat Disord. 2015; 21:205–210. PMID: 25534084.

74. Cruz CD, Cruz F. Spinal cord injury and bladder dysfunction: new ideas about an old problem. ScientificWorldJournal. 2011; 11:214–234. PMID: 21258763.

75. Sahai A, Cortes E, Seth J, Khan MS, Panicker J, Kelleher C, et al. Neurogenic detrusor overactivity in patients with spinal cord injury: evaluation and management. Curr Urol Rep. 2011; 12:404–412. PMID: 21964989.

76. Panicker JN, de Seze M, Fowler CJ. Rehabilitation in practice: neurogenic lower urinary tract dysfunction and its management. Clin Rehabil. 2010; 24:579–589. PMID: 20584864.

77. Sekido N, Jyoraku A, Okada H, Wakamatsu D, Matsuya H, Nishiyama H. A novel animal model of underactive bladder: analysis of lower urinary tract function in a rat lumbar canal stenosis model. Neurourol Urodyn. 2012; 31:1190–1196. PMID: 22473471.

78. Daneshgari F, Leiter EH, Liu G, Reeder J. Animal models of diabetic uropathy. J Urol. 2009; 182(6 Suppl):S8–S13. PMID: 19846143.

79. Aizawa N, Homma Y, Igawa Y. Characteristics of lower urinary tract dysfunction and bladder afferent nerve properties in type 2 diabetic goto-kakizaki rats. J Urol. 2013; 189:1580–1587. PMID: 23103236.

80. Imamura T, Ishizuka O, Ogawa T, Yamagishi T, Yokoyama H, Minagawa T, et al. Muscarinic receptors mediate cold stress-induced detrusor overactivity in type 2 diabetes mellitus rats. Int J Urol. 2014; 21:1051–1058. PMID: 24807830.
81. Saito M, Okada S, Kazuyama E, Satoh I, Kinoshita Y, Satoh K. Pharmacological properties, functional alterations and gene expression of muscarinic receptors in young and old type 2 Goto-Kakizaki diabetic rat bladders. J Urol. 2008; 180:2701–2705. PMID: 18951563.

82. Matsumoto Y, Torimoto K, Matsuyoshi H, Hirayama A, Fujimoto K, Yoshimura N, et al. Long-term effects of diabetes mellitus on voiding function in a new model of type 2 diabetes mellitus, the Spontaneously Diabetic Torii (SDT) rat. Biomed Res. 2009; 30:331–335. PMID: 20051641.

83. Tatemichi S, Tsuchioka K, Yonekubo S, Maruyama K, Kobayashi M. Effects of silodosin, an alpha1a-adrenoceptor antagonist, and distigmine, an acetylcholinesterase inhibitor, and their combined effects on impaired voiding function in zucker diabetic fatty rats. Pharmacology. 2015; 95:285–292. PMID: 26023044.
84. Lowalekar SK, Cristofaro V, Radisavljevic ZM, Yalla SV, Sullivan MP. Loss of bladder smooth muscle caveolae in the aging bladder. Neurourol Urodyn. 2012; 31:586–592. PMID: 22374691.

85. Oshiro T, Miyazato M, Saito S. Relationship between connexin43-derived gap junction proteins in the bladder and age-related detrusor underactivity in rats. Life Sci. 2014; 116:37–42. PMID: 25150797.

86. Hotta H, Morrison JF, Sato A, Uchida S. The effects of aging on the rat bladder and its innervation. Jpn J Physiol. 1995; 45:823–836. PMID: 8713179.

87. Melman A, Tar M, Boczko J, Christ G, Leung AC, Zhao W, et al. Evaluation of two techniques of partial urethral obstruction in the male rat model of bladder outlet obstruction. Urology. 2005; 66:1127–1133. PMID: 16286152.

88. Hashimoto T, Nagabukuro H, Doi T. Effects of the selective acetylcholinesterase inhibitor TAK-802 on the voiding behavior and bladder mass increase in rats with partial bladder outlet obstruction. J Urol. 2005; 174:1137–1141. PMID: 16094081.

89. Matsumoto S, Hanai T, Ohnishi N, Yamamoto K, Kurita T. Bladder smooth muscle cell phenotypic changes and implication of expression of contractile proteins (especially caldesmon) in rats after partial outlet obstruction. Int J Urol. 2003; 10:339–345. PMID: 12757606.

90. Zeng J, Xie K, Jiang C, Mo J, Lindstrom S. Bladder mechanoreceptor changes after artificial bladder outlet obstruction in the anesthetized rat. Neurourol Urodyn. 2012; 31:178–184. PMID: 22038729.

91. Matsumoto S, Hanai T, Uemura H, Levin RM. Effects of chronic treatment with vardenafil, a phosphodiesterase 5 inhibitor, on female rat bladder in a partial bladder outlet obstruction model. BJU Int. 2009; 103:987–990. PMID: 19021615.

92. Somogyi GT, Yokoyama T, Szell EA, Smith CP, de Groat WC, Huard J, et al. Effect of cryoinjury on the contractile parameters of bladder strips: a model of impaired detrusor contractility. Brain Res Bull. 2002; 59:23–28. PMID: 12372544.

93. Kim SJ, Lee DS, Bae WJ, Kim S, Hong SH, Lee JY, et al. Functional and molecular changes of the bladder in rats with crushing injury of nerve bundles from major pelvic ganglion to the bladder: role of RhoA/Rho kinase pathway. Int J Mol Sci. 2013; 14:17511–17524. PMID: 23985824.

94. Hannan JL, Powers SA, Wang VM, Castiglione F, Hedlund P, Bivalacqua TJ. Impaired contraction and decreased detrusor innervation in a female rat model of pelvic neuropraxia. Int Urogynecol J. 2017; 28:1049–1056. PMID: 27987021.

95. Kontani H, Hayashi K. Urinary bladder response to hypogastric nerve stimulation after bilateral resection of the pelvic nerve or spinal cord injury in rats. Int J Urol. 1997; 4:394–400. PMID: 9256330.

96. Kwon D, Minnery B, Kim Y, Kim JH, de Miguel F, Yoshimura N, et al. Neurologic recovery and improved detrusor contractility using muscle-derived cells in rat model of unilateral pelvic nerve transection. Urology. 2005; 65:1249–1253. PMID: 15922415.

97. Ishida T, Shimoda N, Sato K, Ogawa O, Nishizawa O, Kato T. Effects of ischemia on voiding function and nerve growth factor of the rat urinary bladder. Nihon Hinyokika Gakkai Zasshi. 1999; 90:564–571. PMID: 10386056.

98. Ohmura M, Yokoi K, Kondo A, Miyake K, Saito M. Effects of ischemia on the function of the isolated rat detrusor muscle. Hinyokika Kiyo. 1996; 42:111–115. PMID: 8712084.
99. Saito M, Yokoi K, Ohmura M, Kondo A. Effects of ligation of the internal iliac artery on blood flow to the bladder and detrusor function in rat. Int Urol Nephrol. 1998; 30:283–292. PMID: 9696334.

100. Nomiya M, Yamaguchi O, Andersson KE, Sagawa K, Aikawa K, Shishido K, et al. The effect of atherosclerosis-induced chronic bladder ischemia on bladder function in the rat. Neurourol Urodyn. 2012; 31:195–200. PMID: 21905085.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download