Abstract
Underactive bladder (UAB) is a common urologic condition but a complex disease that causes troublesome lower urinary tract symptoms. Currently, management of UAB remains unsatisfactory. Also, many urological diseases can be combined with UAB. In these combined cases, the treatment results may be affected by UAB component. This review focuses on the clinical implications of UAB in patients with common urologic conditions, including bladder outlet obstruction, overactive bladder syndrome and stress urinary incontinence.
Underactive bladder (UAB) as a new concept has become a focus of active research [12345]. Miyazato et al. [1] used the term UAB not only as an equivalent to detrusor underactivity (DU), but also in a broader sense to mirror overactive bladder syndrome (OAB). DU is a urodynamic diagnosis, but no specific parameters have been defined in pressure flow study. The typical urodynamic findings of DU are a low-pressure, poorly sustained detrusor contraction and low flow. Clinical experience and evidence from available urodynamic case series suggest that DU occurs in diverse patient groups, pointing towards the existence of multiple etiological factors. These factors are likely to manifest in DU by disrupting the processes involved in the generation of an effective coordinated voiding contraction. Clinical diagnosis of DU is not easy due to varied symptoms and the absence of a key symptom such as urgency in OAB patients.
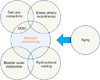
Age-related symptoms such as urinary retention, weak stream, and/or incontinence have been attributed to DU and suggest that DU has age-associated prevalence. One half of elderly men and three-quarters of elderly women with DU have other urologic conditions such as OAB, bladder outlet obstruction (BOO), or stress urinary incontinence (SUI) [6]. As a result, UAB may overlap with BOO, OAB, or SUI (Fig. 1).
In the absence of any specific treatment method for DU, urologists face a dilemma about whether or not they should treat DU patients with other urologic diseases. UAB combined with other urological diseases may exacerbate symptoms and be considered the cause of treatment failure. The present review discusses the clinical implications of UAB in patients with common urologic conditions, including BOO, OAB, and SUI.
The pharmacological treatment of UAB is limited. Theoretically, all the agents that can increase detrusor contractility and/or decrease bladder outlet resistance are useful [7]. Medical treatment of UAB can provide direct stimulation of muscarinic receptors by agonists like bethanechol or carbachol and reduce urethral resistance through smooth muscle relaxation during voiding by alpha blockers. In meta-analysis, using muscarinic receptor agonists remains controversial due to low efficacy and the high prevalence of their side effects [8]. Yamanishi et al. [9] demonstrated that combination of an alpha blocker and a muscarinic receptor agonist is more effective than monotherapy in patients with DU. However, previous studies of medical treatment of UAB excluded patients with BOO and previous studies of medical treatments have been used with limited success, there is no effective pharmacotherapy for UAB with BOO [10].
Surgical treatment is an option for BPH patients who are refractory to medical treatment or have acute urinary retention [11]. Transurethral resection of the prostate (TURP) is an effective surgical procedure based on the concept of removing the whole enlarged adenoma. However, about 30% patients still show insufficient improvement of lower urinary tract symptoms (LUTS) after surgical intervention, despite the resolution of obstruction [1213]. About 25%–30% of patients with BPH also have DU, and that deterioration of detrusor contractility is the cause of surgical failure [14]. Given these findings, some studies have reported no benefit of surgery such as TURP in patients with reduced bladder contractility through preoperative urodynamic study (UDS) [15161718]. Javle et al. [17] demonstrated that the treatment failure rate increased up to 80% in impaired detrusor contractility without BOO. The authors mentioned using the simplified Schäfer nomogram preoperatively to grade obstruction in conjunction with detrusor contractility as a reliable factor predictive of surgical success. Thomas et al. [18] retrospectively analyzed patients diagnosed with and without DU through UDS, and concluded that patients who underwent TURP had no longer-term symptomatic or urodynamic benefit than patients who did not receive treatment. Thus, UDS should be performed in men who are suspected to have DU.
However, recently there have been many reports that lowering the resistance of the outlet may be beneficial, even without BOO, if DU patients have no response to medication or have a high post void residual (PVR). Tanaka et al. [19] evaluated whether detrusor contractility, BOO and detrusor overactivity (DO) affected the outcome of TURP. At 3 months postsurgery, patients with worse preoperative BOO experienced better efficacy after surgery. Of the BOO patients, the proportion who experienced excellent or good overall efficacy was over 70% with or without DU and/or DO. Even in patients with pure DU (without DO and BOO), the overall efficacy was as promising as for those with BOO. Han et al. [20] investigated the effect of TURP in patients with weak bladder contractility without BOO. These patients had improvements in International Prostate Symptom Score (IPSS) and PVR after TURP and 64% of patients were satisfied with TURP. Ou et al. [21] evaluated the efficacy of TURP on BPH with detrusor hypocontractility, defined as a maximum flow rate (Qmax) of less than 10 mL/s with a detrusor pressure of less than 30 cmH2O based on UDS. The authors showed that IPSS as well as mean Qmax, PVR, and maximum detrusor pressure were significantly improved after TURP. Three patients who had urinary retention before TURP were able to void 1 year later. Because such patients with unidentified BOO may benefit from TURP, they concluded that these patients should not be excluded from surgical indications simply based on UDS.
Several studies have shown that resolving obstruction by TURP may be helpful in patients suspected of having DU. Seki et al. [14] examined predictors of the outcome of treatment with TURP in DU patients, defining DU as a bladder contractility index of less than 100 and evaluating the efficacy of TURP through symptoms, quality of life (QoL) and Qmax. If a variable was judged to demonstrate “good or greater improvement”, the surgery was considered a success. The presence of DO was the only factor predictive of improvement in symptoms. The baseline total storage symptoms and QoL index in IPSS as well as the presence of DO were predictive factors for improvement in QoL. The degree of BOO and maximum detrusor pressure at Qmax were independent predictors that correlated with an improvement in Qmax.
Recently, laser prostatectomy has emerged as a safe and effective way to treat BPH [222324]. There have been several current reports on the efficacy of laser prostatectomy such as Holmium laser enucleation of the prostate (HoLEP) or photo selective vaporization of the prostate (PVP) in BPH patients with DU [25262728]. Studies of HoLEP in DU or nonneurogenic acontractile patients reported that all patients were voiding spontaneously with intermittent catheterization for large PVRs at last follow-up. Of those requiring catheterization before surgery, 88.9% of DU patients and 62.5% of acontractile patients were catheter free in long-term follow-up [2629]. There is a study comparing the efficacy of PVP in male patients with BOO but without DU to those with BOO and DU. Patients with BOO and DU had a significantly improved IPSS, Qmax, and PVR compared to prior to PVP. In addition, there was no significant difference when compared with patients with BOO and without DU, so PVP was presented as an option to resolve BOO in DU patients [25].
With respect to comparative studies of TURP and laser prostatectomy, the data are insufficient. Woo et al. [28] carried out a retrospective study comparing TURP with HoLEP for patients with BOO and DU. Both procedures were effective for resolving BOO, but HoLEP showed better efficacy than TURP for improving voiding symptoms, Qmax, PVR, and medication requirements, with the exception of operative time. Cho et al. [27] evaluated the impacts of DU on outcomes of HoLEP or PVP for BPH. Regardless of the existence of DU, both PVP and HoLEP were effective for BPH. However, in patients with DU, improvement in voiding symptoms, Qmax, and voiding efficiency could be superior after HoLEP than after PVP.
UAB component of male LUTS often decrease the effect of treatment. Although BPH surgery is the treatment for BOO, it may also be effective for the patients with DU with LUTS unresponsive to medical treatment. Decreased contractility of detrusor may induce false-negative diagnosis of BOO. The diagnosis of BOO is established with synchronous elevation of detrusor pressure with low flow rate during voiding in UDS. If detrusor pressure cannot be increased against obstruction, that patients can be diagnosed as nonobstruction. In this case, BPH surgery can be effective because it reduces the bladder outlet resistance that was not recognized by UDS.
DU affects 9%–28% of men under the age of 50 years and 48% of those over 70 years undergoing UDS. The prevalence of DU and DO increases with age, and 46.5% of men with DU also have DO or BOO. In women, DU is diagnosed by UDS about 40% of the time and prevalence increases with age, particularly after age 70. DU is accompanied by DO or SUI in 72.6% of women with DU [26]. As a result, UAB is not a pure condition and UAB symptoms overlap with those of OAB. Both are syndromes with shared common symptoms including urgency, frequency, nocturia, and incontinence [30].
OAB and UAB can occur together, which is detrusor overactivity and impaired contractility (DOIC). DOIC paradoxically includes DO during storage but poor detrusor contraction in the voiding phase [31]. DOIC epidemiology is predominantly studied among the elderly, and prevalence estimates vary between reports. In a study that analyzed UDS for patients older than 70 years, the prevalence of DOIC was reported to be 32% for males and 6% for females [32].
There is no standard treatment for patients with DOIC. Liu et al. [33] retrospectively evaluated clinical outcomes of 54 male patients with DOIC. Of those who had received active treatment, 35% received anticholinergics, 10% had anticholinergics and an alpha blocker, 37.5% had an alpha blocker with or without bethanechol, 5% had bethanechol, and 12.5% had surgery based on bothersome symptoms. Overall, 56% of treated patients saw improvement in their symptoms. Thus, clinicians may treat based on the severity of the patient's symptoms. Anticholinergics may be considered carefully in patients with predominant storage symptoms. Periodic follow-up is recommended to ensure that there is no development of urinary retention or increased PVR. A β3-agonist, mirabegron, has been reported to control OAB symptoms safely and effectively in elderly patients [3435]. Although there is no data yet on patients with DOIC, the use of mirabegron can be considered for the administration of anticholinergics in the elderly. Another treatment of option is intravesical onabotulinumtoxinA injection, which is known as an effective and safe treatment option for refractory OAB symptoms [3637]. There is one study of the efficacy and safety of intravesical onabotulinumtoxinA injection in patients with DOIC compared to DO [38]. The therapeutic efficacy lasted for an average of 7 months in patients with DO, and an average of 5 months in DOIC patients (p=0.03). The incidence of adverse events, including urinary retention, increased PVR, and urinary tract infection was comparable, but there was the potential for urinary retention in patients with DOIC. The efficacy was limited for treatment of DOIC, and further clinical studies are required. To improve voiding symptoms, desirable medications will increase bladder contraction or reduce the resistance of the bladder outlet. However, to date, there is no sufficient evidence to recommend muscarinic receptor agonists for the treatment of DU to increase contraction [8]. Sacral neuromodulation (SNM) is thought to alter the afferent pathway to increase parasympathetic activity and affect the urethral and sphincter complex instantiating the guarding reflex to relax the outlet [39]. Recently, Hennessey et al. [40] found that SNM is a potential option for patients with DOIC, treating both DO and impaired contractility components.
Management of DOIC is challenging and involves a carefully individualized plan. Because some treatment options targeting voiding symptoms may potentially aggravate storage symptoms and vice versa, a balanced treatment plan addressing both storage and voiding symptoms is important. Despite having received several kinds of treatments, initiation of clean intermittent catheterization or placement of an indwelling catheter is recommended in patients with chronic urinary retention or large PVR. In the pathophysiological relationships between OAB, DU, and DOIC, Chancellor [41] suggested the following hypothesis: chronic untreated or refractory OAB progresses to DOIC and, ultimately, the development of UAB. Accordingly, it may be beneficial to treat OAB before progression to UAB, because there is no definite treatment in the case of a diagnosis of DOIC or UAB.
Midurethral sling (MUS) placement is a safe and effective treatment for SUI. Theoretically, the MUS procedure does not cause voiding difficulty, but both decreased Qmax and increased voiding pressure after MUS placement have been reported [42]. In addition, several studies have shown that lower Qmax before surgery and patient age are unfavorable predictors for postoperative urinary retention and overall treatment outcome [43444546]. Because patients with DU already have low urine flow, treatment with the MUS procedure may increase the chance of voiding difficulty or urinary retention. Kuo [47] demonstrated such an effect of detrusor function on the therapeutic outcome of MUS for SUI in women. A continence rate of 60% and satisfaction rate of 82% were found in patients with DU or an acontractile detrusor. After the MUS procedure, 36% reported voiding difficulty and 36% had persistent SUI. Kim and Kim [48] found a continence rate of 88% and satisfaction rate after MUS of 71% in patients with DU. They also reported that PVR increased significantly after MUS. Overall, the continence rate after MUS in patients with DU was lower than in patients with normal detrusor function, indicating that DU may be a negative predictor of an unfavorable outcome after MUS. The sling may be loosely applied in patients with persistent SUI, and even adequate tension on the urethra may cause postoperative voiding difficulty in patients with DU.
Because it is important to maintain proper tension of the sling in SUI with DU patients, an adjustable sling could be another option. To date, several tension-control meshes have been introduced that can control additional tension according to the postoperative status of urine leak or voiding difficulty. These meshes include transvaginal adjustable tape (Agency for Medical Innovations, Feldkirch, Austria), Regulation Mechanical External (Remeex; Neomedic International, Terrassa, Spain), and transobturator adjustable tape (TOA) (Agency for Medical Innovations, Feldkirch, Austria). Jo et al. [49] described the success rate for TOA in SUI patients with DU (Qmax <15 mL/s) of 93.3%. Lee et al. [50] performed a multicenter, prospective study of 65 women who underwent TOA due to severe SUI and combined SUI and voiding difficulty (Qmax ≤12 mL/s with a voided volume ≥100 mL). Fourteen patients (21%) underwent loosening and 13 patients (20%) underwent sling tensioning. At 6 months, the cure rate for patients with combined SUI and voiding difficulty was 84.4% and the satisfaction rate was 86.2%. Ko et al. [51] reported the outcome of Remeex applied in SUI patients with DU. The treatment success rate was 81.5% at a mean follow-up period of 38 months. After the Remeex procedure, subjective symptoms improved significantly and Qmax decreased, but there was no difference in PVR compared to baseline. An adjustable sling may be useful in regulating tension at any time, given the possibility of SUI recurrence or worsening of voiding dysfunction in SUI patients with DU. Although there are studies suggesting high objective and subjective cure rates with adjustable slings, prospective randomized trials with long-term follow-up data are still needed.
Although common with age, UAB is a complex disease that can be accompanied by various urological disorders. Whether there is an advantage in resolving the BOO in DU patients remains controversial, but there have been many recent reports of favorable BPH surgery. Management of DOIC is very challenging and should be tailored to each patient's main symptom. The satisfaction of SUI patients with DU after an MUS procedure is good, but the risk of voiding difficulty after surgery suggests that the use of an adjustable sling should be considered.
Obviously, UDS should be performed to confirm the presence of DU in patients with other urologic conditions. Patients with DU should be counseled that the success rate of conventional treatment may be somewhat lower or more difficult than in patients who have normal detrusor contraction.
Figures and Tables
References
1. Miyazato M, Yoshimura N, Chancellor MB. The other bladder syndrome: underactive bladder. Rev Urol. 2013; 15:11–22.
2. Osman NI, Chapple CR, Abrams P, Dmochowski R, Haab F, Nitti V, et al. Detrusor underactivity and the underactive bladder: a new clinical entity? A review of current terminology, definitions, epidemiology, aetiology, and diagnosis. Eur Urol. 2014; 65:389–398.
3. Chapple CR, Osman NI. Crystallizing the definition of underactive bladder syndrome, a common but under-recognized clinical entity. Low Urin Tract Symptoms. 2015; 7:71–76.
4. Aggarwal H, Zimmern PE. Underactive Bladder. Curr Urol Rep. 2016; 17:17.
5. Dewulf K, Abraham N, Lamb LE, Griebling TL, Yoshimura N, Tyagi P, et al. Addressing challenges in underactive bladder: recommendations and insights from the Congress on Underactive Bladder (CURE-UAB). Int Urol Nephrol. 2017; 49:777–785.
6. Jeong SJ, Kim HJ, Lee YJ, Lee JK, Lee BK, Choo YM, et al. Prevalence and clinical features of detrusor underactivity among elderly with lower urinary tract symptoms: a comparison between men and women. Korean J Urol. 2012; 53:342–348.
7. Andersson KE. Detrusor underactivity/underactive bladder: new research initiatives needed. J Urol. 2010; 184:1829–1830.
8. Barendrecht MM, Oelke M, Laguna MP, Michel MC. Is the use of parasympathomimetics for treating an underactive urinary bladder evidence-based? BJU Int. 2007; 99:749–752.
9. Yamanishi T, Yasuda K, Kamai T, Tsujii T, Sakakibara R, Uchiyama T, et al. Combination of a cholinergic drug and an alpha-blocker is more effective than monotherapy for the treatment of voiding difficulty in patients with underactive detrusor. Int J Urol. 2004; 11:88–96.
10. Abraham N, Goldman HB. An update on the pharmacotherapy for lower urinary tract dysfunction. Expert Opin Pharmacother. 2015; 16:79–93.
11. Oelke M, Bachmann A, Descazeaud A, Emberton M, Gravas S, Michel MC, et al. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2013; 64:118–140.
12. Abrams PH. Prostatism and prostatectomy: the value of urine flow rate measurement in the preoperative assessment for operation. J Urol. 1977; 117:70–71.
13. Doll HA, Black NA, McPherson K, Flood AB, Williams GB, Smith JC. Mortality, morbidity and complications following transurethral resection of the prostate for benign prostatic hypertrophy. J Urol. 1992; 147:1566–1573.
14. Seki N, Kai N, Seguchi H, Takei M, Yamaguchi A, Naito S. Predictives regarding outcome after transurethral resection for prostatic adenoma associated with detrusor underactivity. Urology. 2006; 67:306–310.
15. Rollema HJ, Van Mastrigt R. Improved indication and followup in transurethral resection of the prostate using the computer program CLIM: a prospective study. J Urol. 1992; 148:111–115. discussion 5–6.
16. Van Mastrigt R, Rollema HJ. The prognostic value of bladder contractility in transurethral resection of the prostate. J Urol. 1992; 148:1856–1860.
17. Javle P, Jenkins SA, Machin DG, Parsons KF. Grading of benign prostatic obstruction can predict the outcome of transurethral prostatectomy. J Urol. 1998; 160:1713–1717.
18. Thomas AW, Cannon A, Bartlett E, Ellis-Jones J, Abrams P. The natural history of lower urinary tract dysfunction in men: the influence of detrusor underactivity on the outcome after transurethral resection of the prostate with a minimum 10-year urodynamic follow-up. BJU Int. 2004; 93:745–750.
19. Tanaka Y, Masumori N, Itoh N, Furuya S, Ogura H, Tsukamoto T. Is the short-term outcome of transurethral resection of the prostate affected by preoperative degree of bladder outlet obstruction, status of detrusor contractility or detrusor overactivity? Int J Urol. 2006; 13:1398–1404.
20. Han DH, Jeong YS, Choo MS, Lee KS. The efficacy of transurethral resection of the prostate in the patients with weak bladder contractility index. Urology. 2008; 71:657–661.
21. Ou R, Pan C, Chen H, Wu S, Wei X, Deng X, et al. Urodynamically diagnosed detrusor hypocontractility: should transurethral resection of the prostate be contraindicated. Int Urol Nephrol. 2012; 44:35–39.
22. Zhang X, Shen P, He Q, Yin X, Chen Z, Gui H, et al. Different lasers in the treatment of benign prostatic hyperplasia: a network meta-analysis. Sci Rep. 2016; 6:23503.
23. Montorsi F, Naspro R, Salonia A, Suardi N, Briganti A, Zanoni M, et al. Holmium laser enucleation versus transurethral resection of the prostate: results from a 2-center, prospective, randomized trial in patients with obstructive benign prostatic hyperplasia. J Urol. 2004; 172:1926–1929.
24. Li S, Zeng XT, Ruan XL, Weng H, Liu TZ, Wang X, et al. Holmium laser enucleation versus transurethral resection in patients with benign prostate hyperplasia: an updated systematic review with meta-analysis and trial sequential analysis. PLoS One. 2014; 9:e101615.
25. Choi SW, Choi YS, Bae WJ, Kim SJ, Cho HJ, Hong SH, et al. 120 W greenlight HPS laser photoselective vaporization of the prostate for treatment of benign prostatic hyperplasia in men with detrusor underactivity. Korean J Urol. 2011; 52:824–828.
26. Mitchell CR, Mynderse LA, Lightner DJ, Husmann DA, Krambeck AE. Efficacy of holmium laser enucleation of the prostate in patients with non-neurogenic impaired bladder contractility: results of a prospective trial. Urology. 2014; 83:428–432.
27. Cho MC, Ha SB, Park J, Son H, Oh SJ, Kim SW, et al. Impact of detrusor underactivity on surgical outcomes of laser prostatectomy: comparison in serial 12-month follow-up outcomes between potassium-titanyl-phosphate photoselective vaporization of the prostate (PVP) and holmium laser enucleation of the prostate (HoLEP). Urology. 2016; 91:158–166.
28. Woo MJ, Ha YS, Lee JN, Kim BS, Kim HT, Kim TH, et al. Comparison of surgical outcomes between holmium laser enucleation and transurethral resection of the prostate in patients with detrusor underactivity. Int Neurourol J. 2017; 21:46–52.
29. Lomas DJ, Krambeck AE. Long-term efficacy of holmium laser enucleation of the prostate in patients with detrusor underactivity or acontractility. Urology. 2016; 97:208–211.
30. Gammie A, Kaper M, Dorrepaal C, Kos T, Abrams P. Signs and symptoms of detrusor underactivity: an analysis of clinical presentation and urodynamic tests from a large group of patients undergoing pressure flow studies. Eur Urol. 2016; 69:361–369.
31. Resnick NM, Yalla SV. Detrusor hyperactivity with impaired contractile function. An unrecognized but common cause of incontinence in elderly patients. JAMA. 1987; 257:3076–3081.
32. Abarbanel J, Marcus EL. Impaired detrusor contractility in community-dwelling elderly presenting with lower urinary tract symptoms. Urology. 2007; 69:436–440.
33. Liu S, Chan L, Tse V. Clinical outcome in male patients with detrusor overactivity with impaired contractility. Int Neurourol J. 2014; 18:133–137.
34. Wagg A, Nitti VW, Kelleher C, Castro-Diaz D, Siddiqui E, Berner T. Oral pharmacotherapy for overactive bladder in older patients: mirabegron as a potential alternative to antimuscarinics. Curr Med Res Opin. 2016; 32:621–638.
35. Chapple CR, Siddiqui E. Mirabegron for the treatment of overactive bladder: a review of efficacy, safety and tolerability with a focus on male, elderly and antimuscarinic poor-responder populations, and patients with OAB in Asia. Expert Rev Clin Pharmacol. 2017; 10:131–151.
36. Dowson C, Watkins J, Khan MS, Dasgupta P, Sahai A. Repeated botulinum toxin type A injections for refractory overactive bladder: medium-term outcomes, safety profile, and discontinuation rates. Eur Urol. 2012; 61:834–839.
37. Mohee A, Khan A, Harris N, Eardley I. Long-term outcome of the use of intravesical botulinum toxin for the treatment of overactive bladder (OAB). BJU Int. 2013; 111:106–113.
38. Wang CC, Lee CL, Kuo HC. Efficacy and safety of intravesical onabotulinumtoxinA injection in patients with detrusor hyperactivity and impaired contractility. Toxins (Basel). 2016; 8(3):DOI: 10.3390/toxins8030082.
39. Chancellor MB, Chartier-Kastler EJ. Principles of sacral nerve stimulation (SNS) for the treatment of bladder and urethral sphincter dysfunctions. Neuromodulation. 2000; 3:16–26.
40. Hennessey DB, Hoag N, Gani J. Sacral neuromodulation for detrusor hyperactivity with impaired contractility. Neurourol Urodyn. 2017; 03. 27. [Epub]. DOI: 10.1002/nau.23255.
41. Chancellor MB. The overactive bladder progression to underactive bladder hypothesis. Int Urol Nephrol. 2014; 46:Suppl 1. S23–S27.
42. Lukacz ES, Luber KM, Nager CW. The effects of the tension-free vaginal tape on voiding function: a prospective evaluation. Int Urogynecol J Pelvic Floor Dysfunct. 2004; 15:32–38. discussion 8.
43. Hong B, Park S, Kim HS, Choo MS. Factors predictive of urinary retention after a tension-free vaginal tape procedure for female stress urinary incontinence. J Urol. 2003; 170:852–856.
44. Cetinel B, Demirkesen O, Onal B, Akkus E, Alan C, Can G. Are there any factors predicting the cure and complication rates of tension-free vaginal tape. Int Urogynecol J Pelvic Floor Dysfunct. 2004; 15:188–193.
45. Salin A, Conquy S, Elie C, Touboul C, Parra J, Zerbib M, et al. Identification of risk factors for voiding dysfunction following TVT placement. Eur Urol. 2007; 51:782–787. discussion 7.
46. Cho ST, Song HC, Song HJ, Lee YG, Kim KK. Predictors of postoperative voiding dysfunction following transobsturator sling procedures in patients with stress urinary incontinence. Int Neurourol J. 2010; 14:26–33.
47. Kuo HC. Effect of detrusor function on the therapeutic outcome of a suburethral sling procedure using a polypropylene sling for stress urinary incontinence in women. Scand J Urol Nephrol. 2007; 41:138–143.
48. Kim SJ, Kim JC. Influence of preoperative detrusor underactivity on the continence rate and satisfaction after midurethral sling patient with stress urinary incontinence. LUTS: Lower Urinary Tract Symptoms. 2010; 2:95–99.
49. Jo DG, Yang SA, Seo JT. Effects of transobturator adjustable tape sling procedure on the therapeutic outcome in patients with stress urinary incontinence and detrusor underactivity. Int Neurourol J. 2010; 14:20–25.
50. Lee SY, Lee YS, Lee HN, Choo MS, Lee JG, Kim HG, et al. Transobturator adjustable tape for severe stress urinary incontinence and stress urinary incontinence with voiding dysfunction. Int Urogynecol J. 2011; 22:341–346.
51. Ko KJ, Suh YS, Sung HH, Ryu GH, Lee M, Lee KS. Assessing the readjustable sling procedure (Remeex System) for female stress urinary incontinence with detrusor underactivity. Int Neurourol J. 2017; 21:116–120.




 Citation
Citation Print
Print


 XML Download
XML Download