Abstract
Vesicoureteral reflux (VUR) is one of the most common diseases in pediatric urology and classified into primary and secondary VUR. Although posterior urethral valve (PUV) is well known as a cause of the secondary VUR, it is controversial that minor urethral deformity recognized in voiding cystourethrography represents mild end of PUV spectrum and contributes to the secondary VUR. We have been studying for these ten years congenital urethral obstructive lesions with special attention to its urethrographic and endoscopic morphology as well as therapeutic response with transurethral incision. Our conclusion to date is that congenital obstructive lesion in the postero-membranous urethra is exclusively PUV (types 1 and 3) and that severity of obstruction depends on broad spectrum of morphological features recognized in PUV. Endoscopic diagnostic criteria for PUV are being consolidated.
This review article focuses on vesicoureteral reflux (VUR) and the underlying urethral anomaly (mechanical obstruction). The most common anomaly is posterior urethral valve (PUV). However, the diagnosis of PUV is sometimes difficult because of its wide spectrum in terms of severity and morphology. The most typical PUV is presented in neonates with history of prenatal bilateral hydronephrosis or in infants with acute pyelonephritis associated with massive VUR. This group of PUV is easy to be diagnosed by typical findings in voiding cystourethrography (VCUG) and endoscopy for confirming valve like structure in the posterior urethra. In contrast, there is another group of PUV which is elusive and overlooked easily in VCUG even if it contributes to VUR or detrusor overactivity. Until recently there has been no reference standard based on findings in VCUG and endoscopy [12]. Although it is still controversial that these milder PUVs are truly pathological or not, the authors have been trying to establish clinical entity of milder end of PUV spectrum, believing its pathogenesis. It is worth reviewing the wide clinical spectrum of PUV.
The anterior urethral valve is also well known among pediatric urologists, but its incidence is much lower than that of PUV. Therefore, we did not refer them in this article.
Primary VUR is defined as VUR present at birth in which the antireflux mechanism is impaired due to a defect in the ureterovesical junction. The authors suppose that the incidence of VUR with true primary origin might be lower in this context among the total population of so-called primary VUR. Not a small number of patients with “primary” VUR may have an underlying condition in which high detrusor pressure during voiding is a causative factor of VUR. High pressure during storage also contributes to upper urinary tract dilatation and later results in reflux. Young children often prematurely evacuate urine and tend to show discoordinated voiding. A discoordinated voiding pattern is often seen in infants with primary VUR [3].
Another underlying condition that contributes to “apparent” primary VUR might be mild PUV, which is sometimes difficult to detect in ordinary imaging studies because it does not present with conventional findings, such as overt dilatation of the posterior urethra (Fig. 1). Dilatation of the posterior urethra is often transiently observed during the voiding phase of VCUG or such dilatation may be totally absent but segmental narrowing of the bulbomembranous urethra may be a single abnormality. What we have to pay attention further in diagnosing infants is not to misdiagnose physiologic discoordination of the external urethral sphincter as PUV, in which the site of mechanical obstruction is located more distally than physiologic discoordination (Fig. 2). Endoscopic confirmation is recommended if high-grade VUR is associated with suspected PUV. Mild urethral obstructive lesion has long been suggested as a contributing factor in VUR and urinary tract infection (UTI) but has not been systematically investigated. This lack of investigation is due to the lack of reference standard regarding morphological features of posterior urethral obstruction.
No consensus has been reached in regard to this matter. However, in this study, we aimed to evaluate the clinical significance of mild or moderate forms of PUV as a cause of UTI, VUR, and incontinence.
For infants less than 1 year of age with febrile UTI, we adopted a “bottom-up approach” from October 2007 to June 2010. Forty-six infants (32 male and 14 female infants) underwent VCUG. From July 2010 to April 2014, we adopted a “top-down approach” for 79 infants (46 male and 23 female infants), and VCUG was performed in 32 infants. From May 2014 to June 2016, we changed our protocol to a “US-oriented approach,” in which objective findings in ultrasonography suggestive of VUR are considered as an indicator to undergo VCUG. Forty-nine infants (34 male and 15 female infants) were included in this latest protocol, and 13 infants underwent VCUG. In total, 91 infants underwent VCUG, and VUR was observed in 30 infants. PUV was found in 8 infants.
For older children who already had voluntary voiding habit, we performed VCUG for all cases of febrile UTI. From October 2007 to December 2013, 30 children among 44 (19 male and 25 female infants) were shown to have VUR and 5 children were shown to have PUV.
Correspondingly, we identified 60 cases with VUR in children with febrile UTI, and VUR secondary to PUV was screened out in 13 children.
In order to more clearly determine the spectrum of PUVs, we observed an additional 94 children with refractory incontinence who underwent VCUG. Endoscopy eventually confirmed that 54 out of 94 children had PUV.
All of our clinical study introduced in this article were approved by Institutional Review Board in Jichi Medical University (approval number: A 16-073).
In order to improve patient comfort, a 4F or 5F soft, pliable small catheter is inserted into the urethra with the patient in the supine position. For infants and young toddlers, contrast medium is instilled into the bladder until spontaneous voiding occurs regardless of the existing volume. There is no need to remove the catheter during voiding. It is preferred that patients are catheter free. However, this condition is not mandatory. Most importantly, several photographs should be taken during voiding in a supine oblique position to investigate the urethra.
For older children who have the capacity to voluntarily void, a soft, pliable small catheter is used. This should be removed when the patient feels a strong urgency to void. The studied patients urinated in an upright oblique position, and serial photographs were taken for at least several seconds (1–2 images/second) during voiding to avoid missing minor urethral lesions.
It is crucial to take serial photographs during voiding (at least 3–4 images for infants and 5–10 images for older children) to make an accurate diagnosis. Abnormal urethral findings included not only persistently dilated posterior urethra but also a transient urethral kink or angulation of the membranous urethra (Fig. 3). Dilatation of the posterior urethra frequently appeared only at or near maximum urine flow. If the above findings were recognized, endoscopy was indicated. Differential diagnosis between type1 and 3 is always hard to be made by VCUG alone.
Although Young's classification of PUV is well known, Douglas Stephens added a more precise explanation for each type of Young's classification in 1996. Stephens' detailed explanations are considered to be the most useful and easily understood in regard to structural characteristics based on embryology. There are 2 main types of PUV: types 1 and 3. Type 2 was originally defined by Young in 1919 but was later determined to be an overclassification. Type 4 is extraordinarily rare. Therefore, the basic morphological types are types 1 and 3. Embryological origin and structural characteristics are summarized in Table 1.
Type 1 PUV originates from abnormally located Wolffian duct orifices. Stephens described its shape as a valve-like lesion oblique to the urethral axis, the most important finding of which is a connection of the lesion to the verumontanum at the 5 and 7 o'clock positions with the formation of posterolateral folds (Fig. 4A, B). In addition, the verumontanum is larger than normal. In 1992, Dewan et al. [45] introduced a new concept regarding the common characteristics of type 1. He stated that the uniform lesion is not a valve but rather a membrane with a posterior hole. He named type 1 as congenital obstructive posterior urethral membrane (COPUM in short) (Fig. 4B). Accordingly, the main part causing obstruction is located in the anterior wall and is created by anterior fusion of the posterolateral folds. Mild type 1 PUV would not likely be overlooked if this concept was kept in mind, even though typical valve-like lesions are absent in bilateral walls. Needless to say, that reference standard as a normal endoscopic view of male children is very important (Fig. 5).
Type 3 PUV originates from persistent urogenital membrane, and Stephens described its shape as a membrane or diaphragm with a hole present in its center (Fig. 4C). Type 3 lesions are transversely related to the urethral axis. The most important finding to exclude a diagnosis of type 1 is a lack of connection to the verumontanum, which is relatively small in comparison to type 1 (Fig. 4C).
Type 3 can present independently or coincide with type 1 PUV. Cobb's collar is allegedly identical to type 3 PUV. However, it is possible that some past reports have confused Cobb's collar with mild type 1 PUV with or without type 3 PUV.
In order to estimate the severity of obstruction in type 1 PUV using endoscopy (not using VCUG or ultrasonography), we would like to propose our method using a sickle-shaped cold knife. If the cold knife is snagged deeply at the bilateral folds (at the 7 and 5 o'clock positions), we classify it as severe (Fig. 6A). If snagging is evident but shallow, we classify it as moderate (Fig. 6B). Finally, if no snagging is shown in the posterolateral folds but fusion in the anterior wall is evident, we classify it as mild (Fig. 6C). According to our experience, this classification of type 1 PUV is closely related to the extent of local lesion spreading in the posterior membranous urethra. In mild type 1, the anterior lesion is adjacent to the verumontanum and the extent of the local lesion is very limited. In the moderate type, the anterior lesion is not adjacent to the verumontanum but is slightly localized more distally. The verumontanum and the anterior lesion are seen within the same field of view by endoscopy. In the mild type, these two cannot be observed within the same field of view, suggesting that the lesion occupies a longer space. Fig. 7 illustrates our concept. For the purpose of identifying the anterior lesion properly, insertion angle of endoscope is crucial. The tip of the endoscope should be directed toward anterior wall surface and observation be started from the point just distal to the bladder neck to membranous urethra by gradually withdrawing the endoscope. If the anterior lesion is absent (PUV is absent), the mucosa of the anterior wall can be continuously observed throughout endoscope withdrawal. If the anterior lesion is present, this continuous observation is impossible due to sudden interruption of the observation by the presence of proximal slope of anterior lesion, which cannot be caught in the endoscopic view.
After filling the bladder with saline, an incision is made with the suprapubic region compressed to fully dilate the posterior urethra. For type 1 PUV, a major incision is made on the membranous lesion at the 12 o'clock position, and an additional incision was made if necessary on any valvular lesion in the 5 or 7 o'clock position. We strongly recommend the use of a sickle-shaped cold knife instead of a diathermy hot knife. With the sickle-shaped cold knife, it is much easier to control the depth as well as the extent of the incision. For type 3, an incision is made on the membranous lesion in the 12 o'clock position. For both types, the incision on the membranous lesion in the 12 o'clock position was made long and deep enough for complete excision (Fig. 6A; Video clip, Supplementary material). When a cold knife is used, extravasation is very rare. We have never experienced intractable gross hematuria, extravasation of urine, or iatrogenic sphincter damage in our experience with more than 100 patients.
According to the data shown by Toronto Sick Kids Hospital in 2000, Grades 3 and 4 hydronephrosis improved to less than grade 2 in 45% and 29% of the cases, respectively. Grades 3, 4, and 5 VUR improved to less than grade 2 in 100%, 43%, and 40% of the cases, respectively. Even if massive VUR is present in cases of type 1 PUV, more than 40% of such VUR is spontaneously downgraded [6]. Their data were mainly focused on changes of upper urinary tract
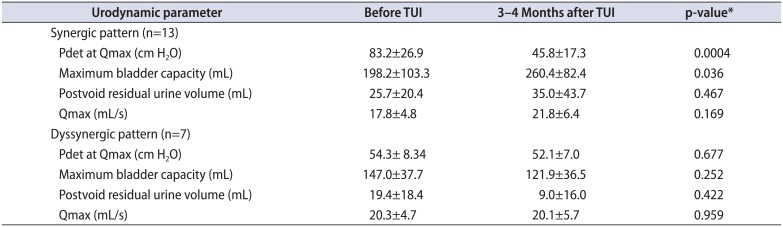
Our data also shows that daytime incontinence which had been refractory to urotherapy and pharmacotherapy improved in more than 80% of the cases by transurethral incision (TUI) of mild to moderate PUV unless dysfunctional voiding is coincident and contributory to dyssynergic voiding (Table 2) [78]. Nakamura et al. [7] pointed out decrement of detrusor pressure at maximal urine flow after TUI as an underlying mechanism related to symptomatic improvement (Table 3).
The reason why endoscopic incision of the anterior lesion recognized in mild PUV is effective for lowering detrusor pressure as well as symptomatic improvement needs to be answered. This question is equal to how such a mild anterior lesion contributes to obstruction. We hypothesize that urethral kinking, which can be observed in these children (preadolescent boys without prostatic development) with mild PUV may be a key for understanding pathophysiology [9]. Hyuga et al. [9] pointed out the changes of urethral morphology recognized in VCUG af ter endoscopic transurethral incision for PUV in boys with intractable daytime urinary incontinence and nocturnal enuresis. VCUG during voiding phase was performed at sequential radiographic spot images (1 image per second) at a 45° angle in oblique standing position. The point at which the angle of the urethra was the smallest during urination was regarded as the minimum urethral angle. The maximum urethral angle during early voiding phase was compared with the minimum urethral angle, and the percentage by which this angle changed was calculated as the flexion rate. Then changes in minimum urethral angle and flexion rate were analyzed before and 3–4 months after TUI. After TUI, the minimum urethral angle on VCUG became more obtuse (before vs. after TUI, respectively: 112.7° vs. 124.5°, p<0.001), the flexion rate decreased (before vs. after TUI, respectively: 11.8% vs. 4.1%, p<0.001).
For the validity of diagnosing and treating mild to moderate PUV, this concept of urethral flexion during voiding in preadolescent childhood (without prostatic development) would be indispensable and better incorporated. It may be an analogy for volume dependent hydronephrosis. The anterior wall lesion would be suspected to cause the urethral flexion due to imbalance of wall distensibility between anterior and posterior aspect of postero-membranous urethra. This hypothesis needs to be further studied and clarified.
The broad spectrum of PUV has gradually become more widely recognized. Among patients with primary VUR (i.e., those without an underlying disease), to date there may at least be some patients with mild concomitant PUV. Therefore, we have been actively diagnosing patients with suspected VCUG using endoscopy.
Accordingly, endoscopic diagnostic criteria for PUV are being consolidated [10]. It is now important to achieve global standardization of image and endoscopic diagnoses for mild PUV as a cause of VUR, UTI, and lower urinary tract symptoms as well.
References
1. de Kort LM, Uiterwaal CS, Beek EJ, Jan Nievels RA, Klijn AJ, de Jong TP. Reliability of voiding cystourethrography to detect urethral obstruction in boys. Urology. 2004; 63:967–971. PMID: 15134990.

2. de Jong TP, Radmayr C, Dik P, Chrzan R, Klijn AJ, de Kort L, et al. Posterior urethral valves: search for a diagnostic reference standard. Urology. 2008; 72:1022–1025. PMID: 18585762.

3. Ichino M, Igawa Y, Seki S, Ishizuka O, Nishizawa O. Natural history and etiology of high pressure voiding in male infants. J Urol. 2007; 178:2561–2565. PMID: 17945306.

4. Dewan PA, Zappala SM, Ransley PG, Duffy PG. Endoscopic reappraisal of the morphology of congenital obstruction of the posterior urethra. Br J Urol. 1992; 70:439–444. PMID: 1450856.

5. Dewan PA, Keenan RJ, Morris LL, Le Quesne GW. Congenital urethral obstruction: Cobb's collar or prolapsed congenital obstructive posterior urethral membrane (COPUM). Br J Urol. 1994; 73:91–95. PMID: 8298906.

6. Farhat W, McLorie G, Capolicchio G, Khoury A, Bägli D, Merguerian PA. Outcomes of primary valve ablation versus urinary tract diversion in patients with posterior urethral valves. Urology. 2000; 56:653–657. PMID: 11018624.

7. Nakamura S, Kawai S, Kubo T, Kihara T, Mori K, Nakai H. Transurethral incision of congenital obstructive lesions in the posterior urethra in boys and its effect on urinary incontinence and urodynamic study. BJU Int. 2011; 107:1304–1311. PMID: 20804485.

8. Nakamura S, Hyuga T, Kawai S, Kubo T, Nakai H. The endoscopic morphological features of congenital posterior urethral obstructions in boys with refractory daytime urinary incontinence and nocturnal enuresis. Eur J Pediatr Surg. 2016; 26:368–375. PMID: 26378483.

9. Hyuga T, Nakamura S, Kawai S, Kubo T, Furukawa R, Aihara T, et al. The changes of urethral morphology recognized in voiding cystourethrography after endoscopic transurethral incision for posterior urethral valve in boys with intractable daytime urinary incontinence and nocturnal enuresis. World J Urol. 2017; 2. 28. [Epub]. DOI: 10.1007/s00345-017-2018-4.

10. Matoka DJ, Marks AJ, Stoltz RS, Maizels M. Utilization of computer enhanced visual learning (CEVL) method improves endoscopic diagnosis of posterior urethral valves (PUV). J Pediatr Urol. 2013; 9:498–502. PMID: 22981142.

SUPPLEMENTARY MATERIAL
Accompanying video can be found in the ‘Urology in Motion’ section of the journal homepage (www.icurology.org). The supplementary video clip can also be accessed by scanning a QR code located on the figure of this article, or be available on YouTube: https://youtu.be/xtDrhVnQhQA.
Fig. 1
In upper case (A–D), physiological discoordination (sphincteric contraction) can be seen transiently in (B) and (C) during stable urinary flow. The arrow shows area of sphincteric contraction. In bottom case (E–H), physiological discoordination occurred suddenly soon after the flow reached maximal flow rate. The arrow shows area of sphincteric contraction.
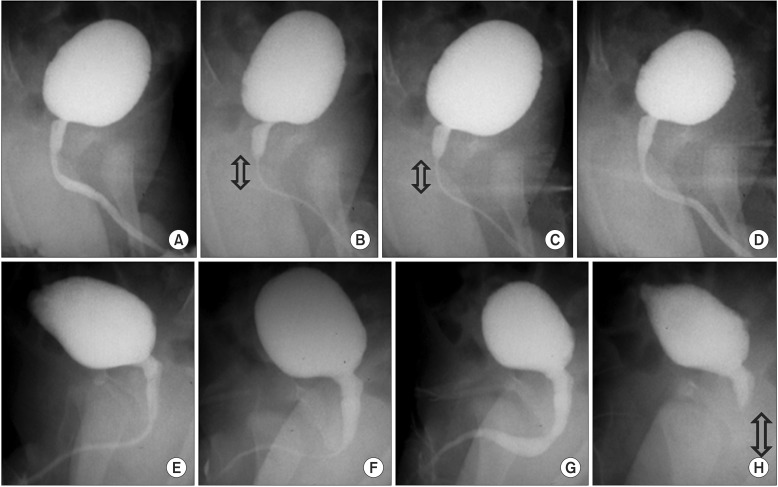
Fig. 2
(A) Overt dilatation of posterior urethra due to posterior urethral valve in 4-month old boy. The arrow depicts the location of the posterior urethral valve. (B) The same case as panel A who presented bilateral vesicoureteral reflux and IRR, resulting from high pressure voiding caused by the posterior urethral valve. IRR, intrarenal reflux.
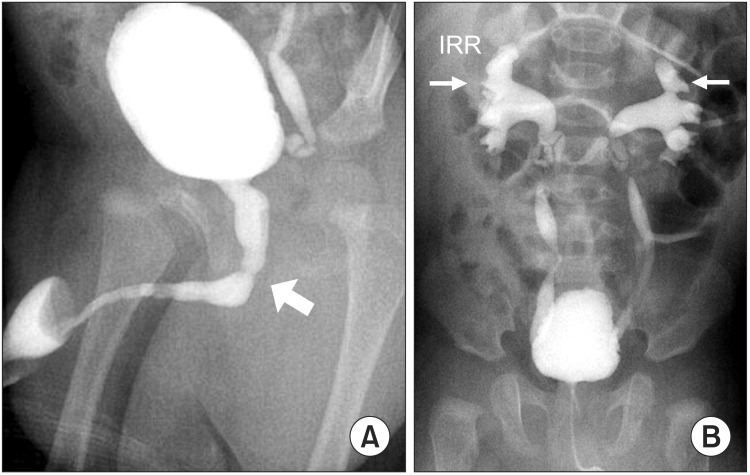
Fig. 3
Transient kinking of posterior urethra recognized in a toddler with right unilateral vesicoureteral reflux. Although the posterior urethra looked rather straight in initial voiding phase (A), it changed the shape by kinking in the mid portion of the posterior urethra (B). Finally, the kinking diminished in the terminal phase of voiding (C).
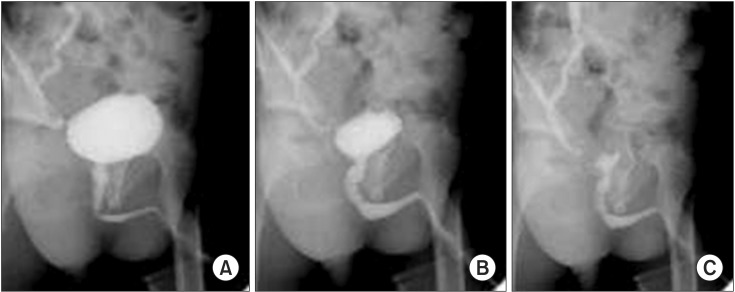
Fig. 4
Endoscopic findings of type 1 posterior urethral valves (PUVs) (A, B) and type 3 PUV (C). Valve like structure (A), membrane like structure (B), and so called “Cobb's collar” seen in type 3 PUV (C). Dotted line depicts margin of the lesion.
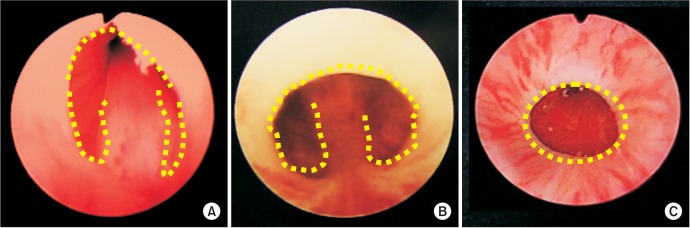
Fig. 5
Normal endoscopic view of membranous urethra in male children. The dotted line shows that distal part connecting to the inferior crest is flat and straight.

Fig. 6
Morphological spectrum of type 1 posterior urethral valve. (A) Severe type showing bilateral valve-like structure, at which cold knife is snagged deeply. (B) Moderate type showing shallow posterolateral folds. (C) Mild type showing subtle posterolateral folds. In contrast to rather thickened lesion in the anterior wall in mild to moderate types, the anterior wall lesion in severe type is thin membrane-like structure. The size of the arrows show the depth of postero-lateral folds. The asterisk indicates verumontanum. Scan this QR code to see the accompanying video, or visit www.icurology.org or https://youtu.be/xtDrhVnQhQA.

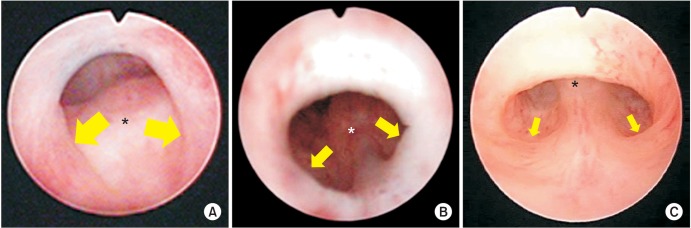
Fig. 7
(A) Lateral view of posterior urethral valve (PUV) type 1, illustrating that milder the lesion bigger the posterior defect (hole) and more distally located anterior fusion. (B) Lateral view of PUV type 1, illustrating that milder the lesion thicker the anterior lesion.
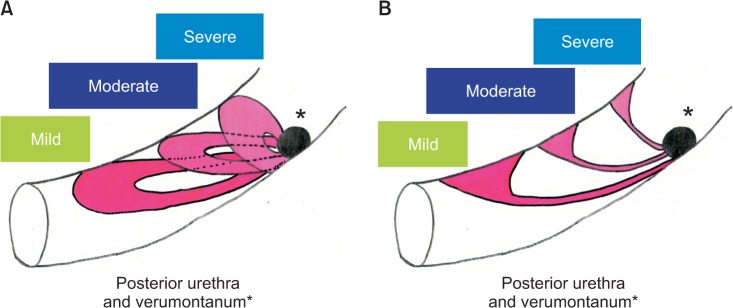
Table 1
Douglas Stephens' description of posterior urethral valve morphology
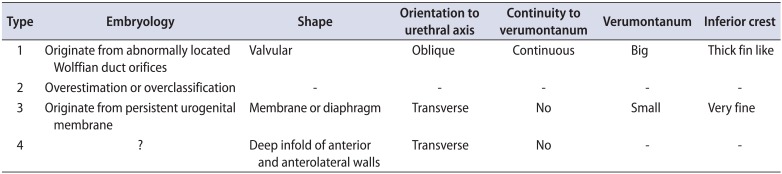
Table 2
Effectiveness of transurethral incision on daytime incontinence and nocturnal enuresis after 3 to 4 months postoperative interval
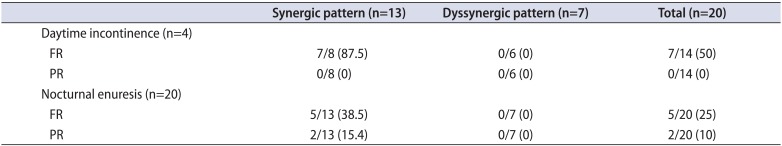
Table 3
Urodynamic parameter change resulting from TUI




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download