Abstract
Purpose
This study aimed to identify the predictors of upgrading and degree of upgrading among patients who have initial Gleason score (GS) 6 treated with robot-assisted radical prostatectomy (RARP).
Materials and Methods
A retrospective review of the data of 359 men with an initial biopsy GS 6, localized prostate cancer who underwent RARP between July 2005 to June 2010 was performed. They were grouped into group 1 (nonupgrade) and group 2 (upgraded) based on their prostatectomy specimen GS. Logistic regression analysis of studied cases identified significant predictors of upgrading and the degree of upgrading after RARP.
Results
The mean age and prostate-specific antigen (PSA) was 63±7.5 years, 8.9±8.77 ng/mL, respectively. Median follow-up was 59 months (interquartile range, 47–70 months). On multivariable analysis, age, PSA, PSA density and ≥2 cores positive were predictors of upgrading with (odds ratio [OR], 1.03; 95% confidence interval [CI], 1.01–1.06; p=0.003; OR, 1.006; 95% CI, 1.01–1.11; p=0.018; OR, 0.65; 95% CI, 0.43–0.98, p=0.04), respectively. On subanalysis, only PSA level of 10–20 ng/mL is associated with upgrading into GS ≥8. They also had lower biochemical recurrence free survival, cancer specific survival, and overall survival (p≤0.001, p=0.003, and p=0.01, respectively).
Due to protracted clinical course of prostate cancer (PCa), clinical guidelines recommend treatment in men with life expectancy of more than 10 years. [1] Appropriate treatment of PCa is largely dependent on the preoperative risk of disease progression among patients. Based on patient's risk stratification, patients may be offered with different treatment modalities. All of these factors should be considered in offering any intervention among patients with localized disease. Nowadays, due to the upstream availability of minimally invasive options low risk PCa are likewise being offered with minimally invasive approach with the aid of robotic system.
In counseling, physicians rely mostly on biopsy Gleason score (GS) of their patients. Discordance between prostate biopsy and prostatectomy GS is not uncommon. Albertsen et al. [2] showed that men 55 to 74 years old with biopsy GS 6 have 18% to 30% risk of dying from PCa in the next 15 years of their lives. It is estimated in previous literatures that 50% of needle biopsy score will be upgraded on final pathology specimen [3]. In our previous study, our results showed that patients treated with radical prostatectomy for clinically low risk disease has 12.9% to 36.4% chance of upgrading [4]. This inaccuracy has serious impact on pretreatment decision-making, predominantly in patients who choose active surveillance or nonsurgical options. Increase in biopsy GS in men with pathological GS 8 to 10 put them on higher risk of death from PCa [5]. Likewise, upgrade in GS put them in increased chances of biochemical recurrence (BCR) [67]. Several articles involving patients treated with open radical prostatectomy have discussed the predictors of GS upgrading [68910]. Nevertheless, no one among them reported the predictor of the degree of upgrading into higher pathologic GS (≥8). To the best of our knowledge this is the first study to report on the predictors of upgrading and the degree of upgrade exclusively on GS 6 patients treated with RARP.
In this study, we report predictors of GS upgrading and degree of upgrading among patients who have an initial GS 6 biopsy treated with RARP.
We retrospectively reviewed records from our prospectively maintained Institutional Review Board-database (approval number: 2014-009-001) of patients who underwent RARP for PCa between July 2005 to June 2010. Patients with clinically localized PCa based on magnetic resonance imaging, digital rectal examination, are included in the study. Twelve-core transrectal ultrasound guided needle biopsy was done on all patients. After excluding patients with nonorgan confined PCa, Prostate-specific antigen (PSA)>20 ng/mL and patients with missing information needed to classify disease risk, 359 men with an initial biopsy GS of 6 were included in the study.
RARP was performed via a transperitoneal approach as described in our previous study [11], using the daVinci Surgical System (Intuitive Surgical, Sunnyvale, CA, USA) with the pelvic lymph node dissection being performed when predicted probability of lymph node metastasis based on clinical information is more than 3% [12].
Patients' clinical and pathological variables: age, body mass index (BMI), prostate volume, PSA, number of core involvement, biopsy and prostatectomy GS were obtained. A dedicated pathologist evaluated the prostatectomy specimens with particular attention to pathologic stage, GS, surgical margins, perineural invasion and angiolymphatic invasion. GS upgrading is considered if initial biopsy GS≤6 increased on prostatectomy specimen to ≥GS 7 [13]. Patients were grouped into group 1 (nonupgrade GS) and group 2 (upgraded GS) and comparative analysis was performed. The primary aim was to evaluate the predictors of GS upgrading and its degree, meanwhile the secondary outcome was to evaluate the oncological outcomes of upgraded GS degrees 7 (3+4, 4+3), and ≥8.
Follow-up with physical examination and PSA quarterly for the first year, semiannually for the second year, and annually thereafter was done. BCR was defined as postoperative PSA≥0.2 ng/mL taken twice at least 6 weeks apart [14].
Statistical analysis was used to analyze demographic and pathological characteristics. Counts of frequencies were expressed as percentages, and continuous data were presented as mean±standard deviation (SD) or median and interquartile range (skewed data). Student t-test was used for continuous variables while chi-square test was used for categorical variables. The probability of BCR, Cancer specific survival (CSS) and overall survival (OS) among upgraded patients were calculated by the Kaplan-Meier analysis. The univariable and multivariable logistic regression models were used to estimate the impact of clinical features on upgrading and degree of upgrading. The IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA) was used for all statistical analyses. A p-value of <0.05 was considered significant, and all p-values were 2-sided.
The mean age of the patient was 63 years old (SD, ±7.5). Mean BMI, prostate volume, and PSA were 24.2±2.6 kg/m2 (interquartile range [IQR], 15.9–30.8 kg/m2), 39.2±20.9 (mL) (IQR, 10.5–164 mL), and 6.8 ng/mL (IQR, 5–10 ng/mL), respectively. Our median follow-up was 59 months (IQR, 47–70 months).
Table 1 summarized the comparative preoperative characteristics. BMI were almost the same for both groups. Patients in the upgraded group were older (p=0.007) and had higher PSA and smaller prostate in volume; 7.7 ng/mL (IQR, 5.2–12.4 ng/mL) and 36.4±16.4 mL, respectively. The mean age of patients in the group 2 was 62.2±7.6 years while it was 64.3±7.3 years for group 1 (p=0.007). Likewise, PSA density is higher in group 2 compared to group 1 with 0.30±0.28 and 0.21±0.14, respectively. Among group 2 patients, 35.9% (52 of 145) had a PSA of 10–20 compared to group 1 with 17.3%. Moreover, 77.2% (112 of 145) of group 2 patients had ≥2 core involvements. More than 25% positive core involvement was seen in 43.4% of patients who upgraded compared to 26.2% who did not upgrade (p=0.001). Group 2 patients had higher pathologic T stage with 44.1% having >T3 (p≤0.001). Positive surgical margin (PSM) was higher in upgraded group with compared to nonupgraded patients (p≤0.009). Significantly, perineural invasion was higher in upgraded group 51.7% versus 19.2% among the nonupgraded group (p≤0.001). Ten percent of group 2 patients showed angiolymphatic invasion compared to 2.3% of group 1 (p=0.002).
Table 2 summarized the comparison among upgraded patients. Patients who upgraded to Gleason score ≥8 had higher preoperative PSA (p=0.000). The results of univariable and multivariable logistic regression analyses were summarized in Table 3. Statistically significant preoperative variables were included in the regression model. Age, PSA, PSA density, clinical T stage, percentage of cores and number of positive had significant result hence further analyzed on regression analysis. Moreover, on multivariable analysis age, PSA, PSA density and >2 cores positive were predictors of upgrading with (odds ratio [OR], 1.03; 95% confidence interval [CI], 1.01–1.06; p=0.003; OR, 1.006; 95% CI, 1.01–1.11; p=0.018; OR, 0.65; 95% CI, 0.43–0.98, p=0.04), respectively were strongest predictors of GS upgrade among clinicopathological variables.
On subanalysis of the predictors of degree of upgrading on Table 4, we found out that only PSA level of 10–20 is significantly associated with the degree of upgrading to GS≥8 (p=0.000). Patients with upgraded GS 8 or more had lower rates of 5 years of BCR-free survival, CSS, and OS (p≤0.001, p=0.003, and p=0.01, respectively) (Figs. 1, 2, 3).
In this study, we have shown that patients preoperative PSA level of 10–20 ng/mL have increased likelihood of upgrading from GS 6 into pathologic GS≥8. Likewise, we report the predictors of upgrading like age, PSA, PSA density, and number of cores among patients who have solely GS 6 on needle biopsy. Knowledge on the impact of GS upgrading is of great importance especially at the point of shared decision-making between the physician and the patient prior to any planned intervention. In a study done by Carlsson et al. [15], they concluded that having surgery as primary treatment for low risk PCa might result in favorable oncologic outcome but may also jeopardize men's potency and continent rate postoperatively. This scenario may have an impact on patients' overall quality of life and great importance is often placed during counseling. At this point, preoperative predictors of upgrading may help urologist in proper counseling of their patients.
Previous reports have discussed that PSA level is a significant predictor of upgrading [1617]. At some point the level of PSA to which upgrading is most likely to occur have been discussed by Colleselli et al. [18]. They reported that patients with PSA range of 2.0–3.9 and 4.0–10.0 ng/mL had a 32.6% and 44% risk of upgrading, respectively. Among the previously mentioned studies, no one has investigated on the predictor of the degree of upgrading among their cohorts. To our knowledge, our study is the first to discuss the predictor of the degree of upgrading on exclusively GS 6 patients on needle biopsy.
Meanwhile, several studies have reported different preoperative predictors of upgrading. Hwang et al. [8], reported that not only PSA but percent positive biopsy core were predictors of upgrading. However their study was limited to a small number of cohort. Similarly, Colleselli et al. [18] found out that tumors are likely to get upgraded when the number of biopsy cores was high even if their PSA is low. Greater than one core positive for cancer on needle biopsy is associated with increased chances of upgrading [9]. In the current study, we found out that patients with more than or equal to 2 cores involved had higher chances of upgrading. This might be explained by the fact that presence of multiple cores increases the likelihood of lymph node invasion and less organ-confined disease (≥pT3). [1920] Thus, even on patients with GS 6 on biopsy risk of upgrading is increased whenever multiple cores are present.
In a cohort of 2,771 men, the group of Serkin et al. [21] reported that patients with higher PSA density were likely to upgrade. And this could also happen even if they are eligible for active surveillance [22]. In support to their findings, PSA density was also a significant predictor of overall upgrading among our patients. However, when PSA density was entered on the model for predicting degree of upgrading it has lost its significance. This might be due to the small number of patients who upgraded to ≥8. Meanwhile, prostate volume has long been a point of debate; several reports have stated that prostate volume is a significant predictor of upgrading among patients who underwent radical prostatectomy [102324]. There seems to be an inverse relationship between prostate size and chances of upgrading which was also evident in our current study.
On the other hand, patients' age showed to have an impact on upgrading in our cohort. This may be explained by the fact that as man ages PSA level tends to increase stepwise. This was also evident in the cohort of 25,858 patients reported by Caster et al. [25], that older age was associated with GS upgrading and pathologically advanced disease.
Difference between biopsy and prostatectomy specimen GS has a significant impact on BCR. Corcoran et al. [6] reported that change in GS on prostatectomy was a predictor of BCR. Moreover, Boorjian et al. [5] also found out that upgrading in GS was associated with BCR and progression to systemic disease, though their study included all GS. Likewise, our findings suggest that BCR was notably higher among patients who upgraded into higher GS in support of the aforementioned studies. Badani et al. [26] reported in their series of 2,766 patients a BCR of 7.3% however its median follow-up was only 22 months. On the other hand, Menon et al. [7], did a study with a median follow-up of 60.2 months however, their study included all GS. In our current study, we reported the oncologic outcome of our cohort at a median follow-up of 59 months for specifically GS 6 patients alone after RARP. This reflects a more realistic oncologic behaviour of patients who were initially GS 6 on biopsy but subsequently changed on pathologic specimen.
Upgraded group of patients have had significant differences in PSM, extraprostatic extension, seminal vesicle invasion, and lymphovascular invasion [27]. Accordingly, the rate of extraprostatic extension, and PSM were noted to be higher among patients who have GS upgrading during radical prostatectomy [9]. In concordance, our results also showed that upgraded group had higher PSM, perineural invasion and angiolymphatic invasion compared to nonupgrade group depicting probably a more aggressive disease.
There are several limitations in our study. First of all, this study was analyzed in a retrospective manner. Small number of patients which represents a higher degree of upgrading from GS 6 is one of its main limitation. Comparative analysis of patients with originally GS ≥8 from biopsy and those who upgraded from GS 6 to ≥8 is likewise recommended. A longer follow-up maybe needed to portray satisfactory oncologic impact of upgrading to clinical course and disease progression of patients with initially GS 6 treated with radical prostatectomy. Despite these limitations, our study showed that patients with higher preoperative PSA level has increased risk of upgrading to higher degree of GS which should be discussed thoroughly before offering any treatment options to patients.
Patients with GS 6 on biopsy with PSA level of 10–20 ng/mL carries an increased risk of upgrading into higher pathologic GS (≥8) with poorer oncologic outcome. Presence of multiple cores, high PSA density increases the likelihood of upgrading. We recommend strict counseling especially in choosing treatment options between surgical and nonsurgical for patients with GS 6 on biopsy whenever these parameters are present.
Figures and Tables
Table 1
Patients' characteristics
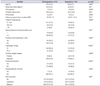
Table 2
Comparison of upgraded Gleason score 6 patients

Table 3
Logistic regression analysis of predictive factors associated with overall upgrading

Table 4
Univariable analysis of the predictor of degree of upgrading to Gleason score≥8

References
1. Mohler J, Bahnson RR, Boston B, Busby JE, D'Amico A, Eastham JA, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2010; 8:162–200.
2. Albertsen PC, Hanley JA, Gleason DF, Barry MJ. Competing risk analysis of men aged 55 to 74 years at diagnosis managed conservatively for clinically localized prostate cancer. JAMA. 1998; 280:975–980.
3. Cohen MS, Hanley RS, Kurteva T, Ruthazer R, Silverman ML, Sorcini A, et al. Comparing the Gleason prostate biopsy and Gleason prostatectomy grading system: the Lahey Clinic Medical Center experience and an international meta-analysis. Eur Urol. 2008; 54:371–381.
4. Lim SK, Kim KH, Shin TY, Chung BH, Hong SJ, Choi YD, et al. Yonsei criteria: a new protocol for active surveillance in the era of robotic and local ablative surgeries. Clin Genitourin Cancer. 2013; 11:501–507.
5. Boorjian SA, Karnes RJ, Crispen PL, Rangel LJ, Bergstralh EJ, Sebo TJ, et al. The impact of discordance between biopsy and pathological Gleason scores on survival after radical prostatectomy. J Urol. 2009; 181:95–104.
6. Corcoran NM, Hong MK, Casey RG, Hurtado-Coll A, Peters J, Harewood L, et al. Upgrade in Gleason score between prostate biopsies and pathology following radical prostatectomy significantly impacts upon the risk of biochemical recurrence. BJU Int. 2011; 108(8 Pt 2):E202–E210.
7. Menon M, Bhandari M, Gupta N, Lane Z, Peabody JO, Rogers CG, et al. Biochemical recurrence following robot-assisted radical prostatectomy: analysis of 1384 patients with a median 5-year follow-up. Eur Urol. 2010; 58:838–846.
8. Hwang I, Lim D, Jeong YB, Park SC, Noh JH, Kwon DD, et al. Upgrading and upstaging of low-risk prostate cancer among Korean patients: a multicenter study. Asian J Androl. 2015; 17:811–814.
9. Sarici H, Telli O, Yigitbasi O, Ekici M, Ozgur BC, Yuceturk CN, et al. Predictors of Gleason score upgrading in patients with prostate biopsy Gleason score ≤6. Can Urol Assoc J. 2014; 8:E342–E346.
10. Kim KH, Lim SK, Shin TY, Lee JY, Chung BH, Rha KH, et al. Upgrading of Gleason score and prostate volume: a clinicopathological analysis. BJU Int. 2013; 111:1310–1316.
11. Rha KH. Robot-assisted laparoscopic radical prostatectomy. Korean J Urol. 2009; 50:97–104.
12. Cagiannos I, Karakiewicz P, Eastham JA, Ohori M, Rabbani F, Gerigk C, et al. A preoperative nomogram identifying decreased risk of positive pelvic lymph nodes in patients with prostate cancer. J Urol. 2003; 170:1798–1803.
13. King CR, McNeal JE, Gill H, Presti JC Jr. Extended prostate biopsy scheme improves reliability of Gleason grading: implications for radiotherapy patients. Int J Radiat Oncol Biol Phys. 2004; 59:386–391.
14. Cookson MS, Aus G, Burnett AL, Canby-Hagino ED, D'Amico AV, Dmochowski RR, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007; 177:540–545.
15. Carlsson S, Jäderling F, Wallerstedt A, Nyberg T, Stranne J, Thorsteinsdottir T, et al. Oncological and functional outcomes 1 year after radical prostatectomy for very-low-risk prostate cancer: results from the prospective LAPPRO trial. BJU Int. 2016; 118:205–212.
16. Dong F, Jones JS, Stephenson AJ, Magi-Galluzzi C, Reuther AM, Klein EA. Prostate cancer volume at biopsy predicts clinically significant upgrading. J Urol. 2008; 179:896–900.
17. Hong SK, Han BK, Lee ST, Kim SS, Min KE, Jeong SJ, et al. Prediction of Gleason score upgrading in low-risk prostate cancers diagnosed via multi (> or = 12)-core prostate biopsy. World J Urol. 2009; 27:271–276.
18. Colleselli D, Pelzer AE, Steiner E, Ongarello S, Schaefer G, Bartsch G, et al. Upgrading of Gleason score 6 prostate cancers on biopsy after prostatectomy in the low and intermediate tPSA range. Prostate Cancer Prostatic Dis. 2010; 13:182–185.
19. Ellis CL, Walsh PC, Partin AW, Epstein JI. Multiple cores of Gleason score 6 correlate with favourable findings at radical prostatectomy. BJU Int. 2013; 111:E306–E309.
20. Kim SJ, Park CM, Seong KT, Kim SY, Kim HK, Park JY. pT3 predictive factors in patients with a Gleason score of 6 in prostate biopsies. Korean J Urol. 2011; 52:598–602.
21. Serkin FB, Soderdahl DW, Cullen J, Chen Y, Hernandez J. Patient risk stratification using Gleason score concordance and upgrading among men with prostate biopsy Gleason score 6 or 7. Urol Oncol. 2010; 28:302–307.
22. Jin BS, Kang SH, Kim DY, Oh HG, Kim CI, Moon GH, et al. Pathological upgrading in prostate cancer patients eligible for active surveillance: Does prostate-specific antigen density matter? Korean J Urol. 2015; 56:624–629.
23. Turley RS, Hamilton RJ, Terris MK, Kane CJ, Aronson WJ, Presti JC Jr, et al. Small transrectal ultrasound volume predicts clinically significant Gleason score upgrading after radical prostatectomy: results from the SEARCH database. J Urol. 2008; 179:523–527.
24. Epstein JI, Feng Z, Trock BJ, Pierorazio PM. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur Urol. 2012; 61:1019–1024.
25. Caster JM, Falchook AD, Hendrix LH, Chen RC. Risk of pathologic upgrading or locally advanced disease in early prostate cancer patients based on biopsy Gleason score and PSA: a population-based study of modern patients. Int J Radiat Oncol Biol Phys. 2015; 92:244–251.
26. Badani KK, Kaul S, Menon M. Evolution of robotic radical prostatectomy: assessment after 2766 procedures. Cancer. 2007; 110:1951–1958.
27. Müntener M, Epstein JI, Hernandez DJ, Gonzalgo ML, Mangold L, Humphreys E, et al. Prognostic significance of Gleason score discrepancies between needle biopsy and radical prostatectomy. Eur Urol. 2008; 53:767–775.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download