1. Colella J, Kochis E, Galli B, Munver R. Urolithiasis/nephrolithiasis: what's it all about? Urol Nurs. 2005; 25:427–428. 475449PMID:
16438249.
2. Trinchieri A. Epidemiology of urolithiasis: an update. Clin Cases Miner Bone Metab. 2008; 5:101–106. PMID:
22460989.
3. Wooldridge AA, Seahorn TL, Williams J, Taylor HW, Oliver JL, Kim DY, et al. Chronic renal failure associated with nephrolithiasis, ureterolithiasis, and renal dysplasia in a 2-year-old quarter horse gelding. Vet Radiol Ultrasound. 1999; 40:361–364. PMID:
10463829.

4. Miller OF, Kane CJ. Time to stone passage for observed ureteral calculi: a guide for patient education. J Urol. 1999; 162(3 Pt 1):688–690. PMID:
10458343.

5. Dellabella M, Milanese G, Muzzonigro G. Randomized trial of the efficacy of tamsulosin, nifedipine and phloroglucinol in medical expulsive therapy for distal ureteral calculi. J Urol. 2005; 174:167–172. PMID:
15947613.

6. Gratzke C, Uckert S, Kedia G, Reich O, Schlenker B, Seitz M, et al. In vitro effects of PDE5 inhibitors sildenafil, vardenafil and tadalafil on isolated human ureteral smooth muscle: a basic research approach. Urol Res. 2007; 35:49–54. PMID:
17102958.

7. Al-Aown A, Kyriazis I, Kallidonis P, Sakellaropoulos G, Vrettos T, Perimenis P, et al. Vardenafil effect on ureteric smooth muscle: in vitro study in porcine model. J Endourol. 2011; 25:505–509. PMID:
21401398.

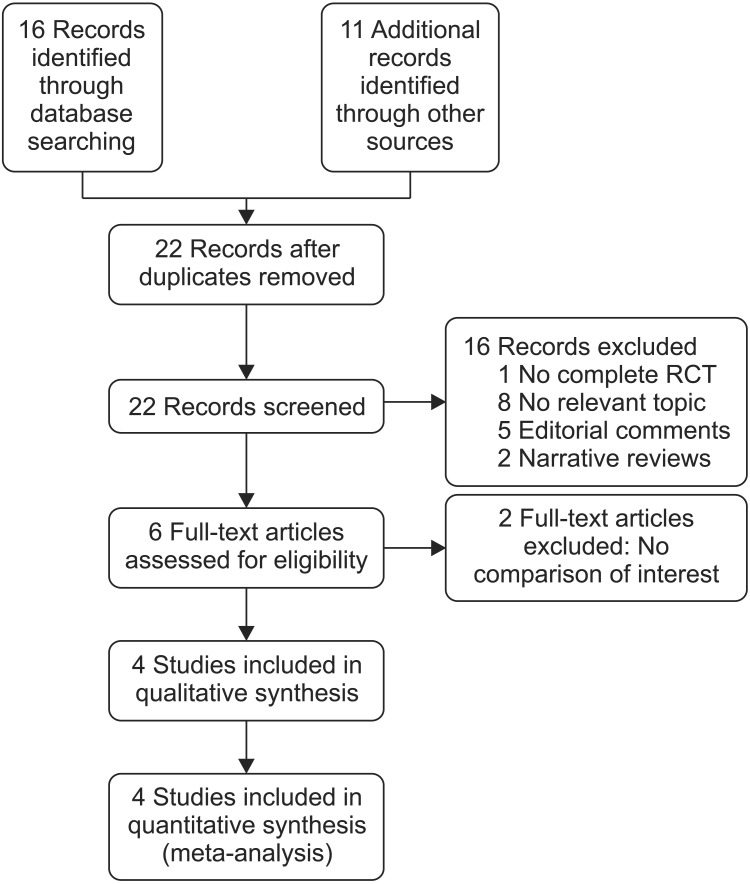
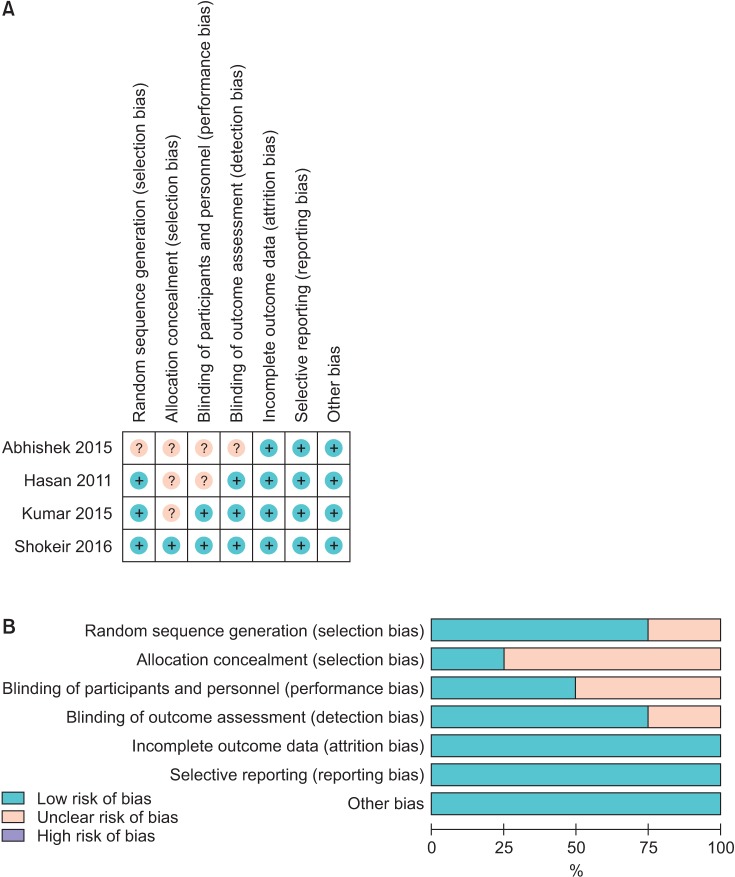
8. Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration;2011. updated 2011 Mar. cited 2016 Nov 5. Available from:
http://handbook.cochrane.org.
9. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–188. PMID:
3802833.

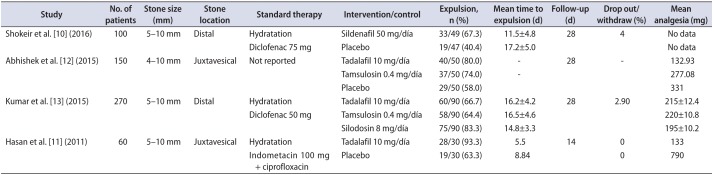
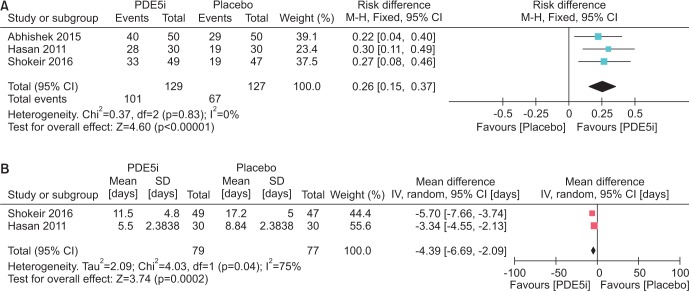
10. Shokeir AA, Tharwat MA, Abolazm AE, Harraz A. Sildenafil citrate as a medical expulsive therapy for distal ureteric stones: A randomised double-blind placebo-controlled study. Arab J Urol. 2016; 14:1–6. PMID:
26966585.

11. Hasan HF, Jaffal WN, Al-Hossona HA. The role of tadalafil in lower ureteric stone expulsion. Iraqi Postgrad Med J. 2011; 10:24–32.
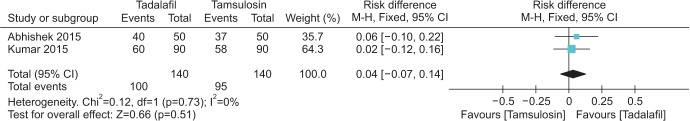
12. Abhishek L, Shashikant N, Arvind G, Ravindra S, Mahesh D. Comparison of tadalafil and tamsulosin in medical expulsive therapy for ureteric calculus: prospective, randomized, placebo controlled study [abstract]. Indian J Urol. 2015; 31(Suppl 1):S39. Abstract No. CKP 03.
13. Kumar S, Jayant K, Agrawal MM, Singh SK, Agrawal S, Parmar KM. Role of tamsulosin, tadalafil, and silodosin as the medical expulsive therapy in lower ureteric stone: a randomized trial (a pilot study). Urology. 2015; 85:59–63. PMID:
25530364.

14. Pickard R, Starr K, MacLennan G, Lam T, Thomas R, Burr J, et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet. 2015; 386:341–349. PMID:
25998582.

15. Sur RL, Shore N, L'Esperance J, Knudsen B, Gupta M, Olsen S, et al. Silodosin to facilitate passage of ureteral stones: a multi-institutional, randomized, double-blinded, placebo-controlled trial. Eur Urol. 2015; 67:959–964. PMID:
25465978.

16. Taher A, Schulz-Knappe P, Meyer M, Truss M, Forssmann WG, Stief CG, et al. Characterization of cyclic nucleotide phosphodiesterase isoenzymes in the human ureter and their functional role in vitro. World J Urol. 1994; 12:286–291. PMID:
7866426.

17. Truss MC, Uckert S, Stief CG, Forssmann WG, Jonas U. Cyclic nucleotide phosphodiesterase (PDE) isoenzymes in the human detrusor smooth muscle. II. Effect of various PDE inhibitors on smooth muscle tone and cyclic nucleotide levels in vitro. Urol Res. 1996; 24:129–134. PMID:
8839479.

18. Kumar S, Jayant K, Agrawal S, Singh SK. Comparative efficacy of tamsulosin versus tamsulosin with tadalafil in combination with prednisolone for the medical expulsive therapy of lower ureteric stones: a randomized trial. Korean J Urol. 2014; 55:196–200. PMID:
24648875.

19. Jayant K, Agrawal R, Agrawal S. Tamsulosin versus tamsulosin plus tadalafil as medical expulsive therapy for lower ureteric stones: a randomized controlled trial. Int J Urol. 2014; 21:1012–1015. PMID:
24894533.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download