1. World Health Organization. Global prevalence and incidence of selected curable sexually transmitted infections: overview and estimates. Geneva: World Health Organization;2001.
2. Cai T, Wagenlehner FM, Mondaini N, D'Elia C, Meacci F, Migno S, et al. Effect of human papillomavirus and Chlamydia trachomatis co-infection on sperm quality in young heterosexual men with chronic prostatitis-related symptoms. BJU Int. 2014; 113:281–287. PMID:
23906072.
3. Wagenlehner FM, Naber KG, Weidner W. Chlamydial infections and prostatitis in men. BJU Int. 2006; 97:687–690. PMID:
16536754.

4. Ouzounova-Raykova V, Ouzounova I, Mitov IG. May Chlamydia trachomatis be an aetiological agent of chronic prostatic infection? Andrologia. 2010; 42:176–181. PMID:
20500746.

5. Mazzoli S, Cai T, Rupealta V, Gavazzi A, Castricchi Pagliai R, Mondaini N, et al. Interleukin 8 and anti-chlamydia trachomatis mucosal IgA as urogenital immunologic markers in patients with C. trachomatis prostatic infection. Eur Urol. 2007; 51:1385–1393. PMID:
17107749.

6. Mazzoli S, Cai T, Addonisio P, Bechi A, Mondaini N, Bartoletti R. Chlamydia trachomatis infection is related to poor semen quality in young prostatitis patients. Eur Urol. 2010; 57:708–714. PMID:
19482415.

7. Cai T, Pisano F, Magri V, Verze P, Mondaini N, D'Elia C, et al. Chlamydia trachomatis infection is related to premature ejaculation in chronic prostatitis patients: results from a cros-ssectional study. J Sex Med. 2014; 11:3085–3092. PMID:
25256084.
8. Krieger JN, Nyberg L Jr, Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999; 282:236–237. PMID:
10422990.

9. Abramson JH, Abramson ZH. Survey methods in community medicine: epidemiological research, programme evaluation, clinical trials. 5th ed. London: Livingstone;2000.
10. Krieger JN, McGonagle LA. Diagnostic considerations and interpretation of microbiological findings for evaluation of chronic prostatitis. J Clin Microbiol. 1989; 27:2240–2244. PMID:
2584375.

11. Naber KG, Bergman B, Bishop MC, Bjerklund-Johansen TE, Botto H, Lobel B, et al. EAU guidelines for the management of urinary and male genital tract infections. Urinary Tract Infection (UTI) Working Group of the Health Care Office (HCO) of the European Association of Urology (EAU). Eur Urol. 2001; 40:576–588. PMID:
11752870.
12. Bartoletti R, Cai T, Mondaini N, Dinelli N, Pinzi N, Pavone C, et al. Prevalence, incidence estimation, risk factors and characterization of chronic prostatitis/chronic pelvic pain syndrome in urological hospital outpatients in Italy: results of a multicenter case-control observational study. J Urol. 2007; 178:2411–2415. PMID:
17937946.

13. Nickel JC, Alexander RB, Schaeffer AJ, Landis JR, Knauss JS, Propert KJ, et al. Leukocytes and bacteria in men with chronic prostatitis/chronic pelvic pain syndrome compared to asymptomatic controls. J Urol. 2003; 170:818–822. PMID:
12913707.

14. Giubilei G, Mondaini N, Crisci A, Raugei A, Lombardi G, Travaglini F, et al. The Italian version of the National Institutes of Health Chronic Prostatitis Symptom Index. Eur Urol. 2005; 47:805–811. PMID:
15925077.

15. Badía X, García-Losa M, Dal-Ré R. Ten-language translation and harmonization of the International Prostate Symptom Score: developing a methodology for multinational clinical trials. Eur Urol. 1997; 31:129–140. PMID:
9076454.
16. Cappelleri JC, Rosen RC, Smith MD, Mishra A, Osterloh IH. Diagnostic evaluation of the erectile function domain of the International Index of Erectile Function. Urology. 1999; 54:346–351. PMID:
10443736.

17. Apolone G, Mosconi P. The Italian SF-36 Health Survey: translation, validation and norming. J Clin Epidemiol. 1998; 51:1025–1036. PMID:
9817120.
18. Symonds T, Perelman MA, Althof S, Giuliano F, Martin M, May K, et al. Development and validation of a premature ejaculation diagnostic tool. Eur Urol. 2007; 52:565–573. PMID:
17275165.

19. Nickel JC, Downey J, Hunter D, Clark J. Prevalence of prostatitis-like symptoms in a population based study using the National Institutes of Health chronic prostatitis symptom index. J Urol. 2001; 165:842–845. PMID:
11176483.

20. Park H, Sim SM, Lee G. The presence of Chlamydia is associated with increased leukocyte counts and pain severity in men with chronic pelvic pain syndrome. Urology. 2015; 85:574–579. PMID:
25733268.

21. Koroku M, Kumamoto Y, Hirose T. A study on the role of Chlamydia trachomatis in chronic prostatitis--analysis of anti-Chlamydia trachomatis specific IgA in expressed prostate secretion by western-blotting method. Kansenshogaku Zasshi. 1995; 69:426–437. PMID:
7751752.
22. Nickel JC, Downey JA, Nickel KR, Clark JM. Prostatitis-like symptoms: one year later. BJU Int. 2002; 90:678–681. PMID:
12410746.

23. Turner JA, Ciol MA, Von Korff M, Berger R. Prognosis of patients with new prostatitis/pelvic pain syndrome episodes. J Urol. 2004; 172:538–541. PMID:
15247724.

24. Skerk V, Schönwald S, Krhen I, Banaszak A, Begovac J, Strugar J, et al. Comparative analysis of azithromycin and ciprofloxacin in the treatment of chronic prostatitis caused by Chlamydia trachomatis. Int J Antimicrob Agents. 2003; 21:457–462. PMID:
12727080.

25. Serefoglu EC, Yaman O, Cayan S, Asci R, Orhan I, Usta MF, et al. The comparison of premature ejaculation assessment questionnaires and their sensitivity for the four premature ejaculation syndromes: results from the Turkish society of andrology sexual health survey. J Sex Med. 2011; 8:1177–1185. PMID:
21269396.

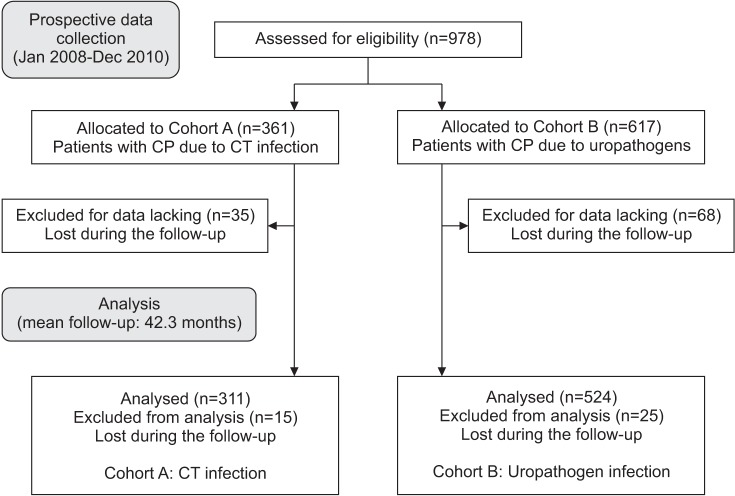
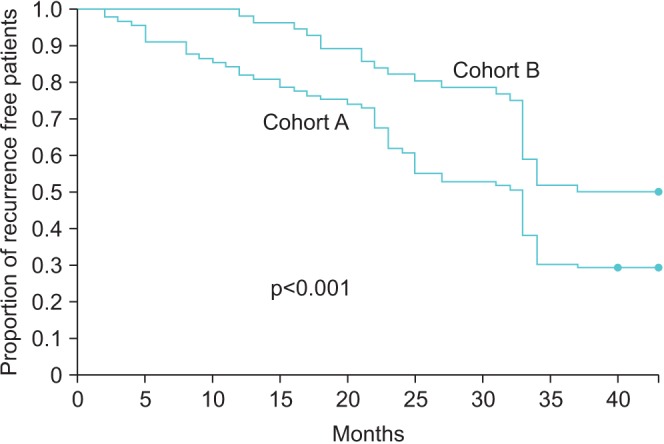
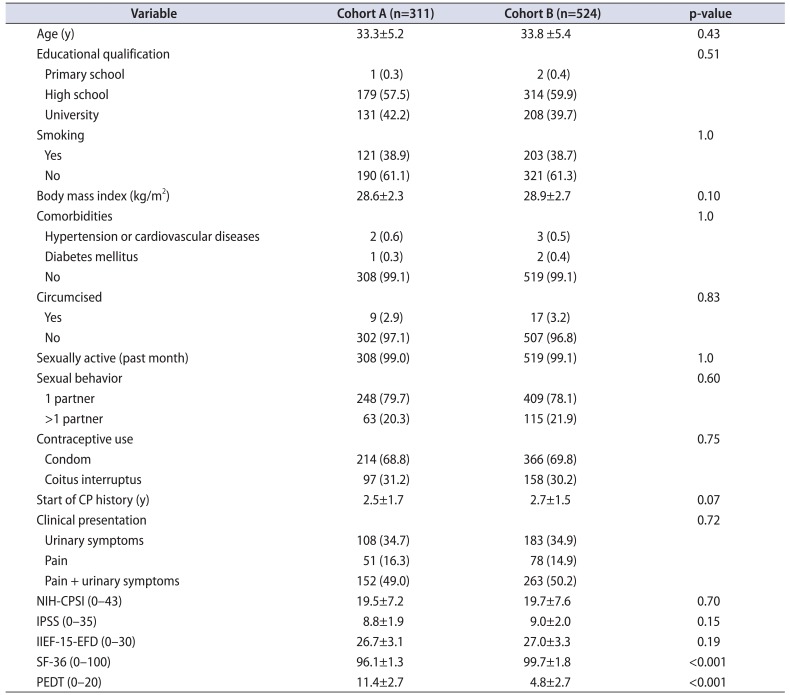
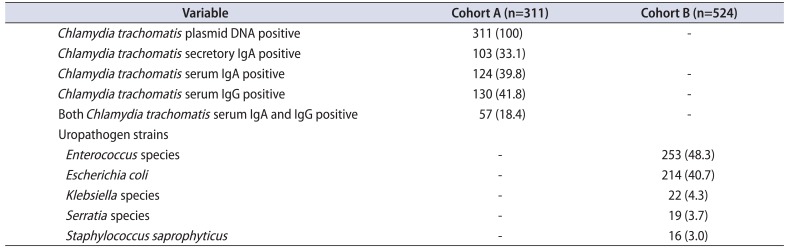




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download