Abstract
Purpose
Urosepsis implies clinically evident severe infection of urinary tract with features of systemic inflammatory response syndrome (SIRS). We validate the role of a single Acute Physiology and Chronic Health Evaluation II (APACHE II) score at 24 hours after admission in predicting mortality in urosepsis.
Materials and Methods
A prospective observational study was done in 178 patients admitted with urosepsis in the Department of Urology, in a tertiary care institute from January 2015 to August 2016. Patients >18 years diagnosed as urosepsis using SIRS criteria with positive urine or blood culture for bacteria were included. At 24 hours after admission to intensive care unit, APACHE II score was calculated using 12 physiological variables, age and chronic health.
Results
Mean±standard deviation (SD) APACHE II score was 26.03±7.03. It was 24.31±6.48 in survivors and 32.39±5.09 in those expired (p<0.001). Among patients undergoing surgery, mean±SD score was higher (30.74±4.85) than among survivors (24.30±6.54) (p<0.001). Receiver operating characteristic (ROC) analysis revealed area under curve (AUC) of 0.825 with cutoff 25.5 being 94.7% sensitive and 56.4% specific to predict mortality. Mean±SD score in those undergoing surgery was 25.22±6.70 and was lesser than those who did not undergo surgery (28.44±7.49) (p=0.007). ROC analysis revealed AUC of 0.760 with cutoff 25.5 being 94.7% sensitive and 45.6% specific to predict mortality even after surgery.
Urosepsis implies clinically evident severe infection of the urinary tract with features of systemic inflammatory response syndrome (SIRS) [1]. Though many factors and scoring systems have been identified to diagnose and prognosticate urosepsis, they are not accurate. Prognostication of urosepsis is important in the intensive care unit (ICU) to determine the need for defining the severity, timing of surgical intervention if necessary and predict mortality. Of the existing severity-of-disease classif ication systems available for ICU patients, a single Acute Physiology and Chronic Health Evaluation II (APACHE II) score at 24 hours after admission has been found to be efficient in predicting mortality [23]. The advantages of APACHE II score include its ability to prognosticate sepsis with a single assessment at 24 hours and its components being routine parameters being monitored in the ICU. To the best of our knowledge, it has not been validated for urosepsis yet. We aim to validate the role of APACHE II score in urosepsis patients in this study.
This was hospital based, prospective observational study done in 178 patients admitted with a diagnosis of urosepsis in the Department of Urology, in a tertiary care institute, in South India. After obtaining approval from the Institute Research Council and Ethics committee, the study was conducted from January 2015 to August 2016 (approval number: INU/RRC/01/2015-16). Written informed consent was obtained from each patient for inclusion in this study.
All consecutive consenting patients more than 18 years diagnosed as urosepsis using SIRS criteria were evaluated and included in the study [45]. All included patients had growth on urine or blood culture. Patients aged <18 years, negative blood and urine culture, positive sputum culture, other sources of infections like pneumonia or bloodstream sepsis in patients on hemodialysis catheters during evaluation, having incomplete set of investigations or physiological variables and those who had less than 24-hour stay in ICU were excluded.
Patients were evaluated in the Emergency Department and admitted to the ICU after initial evaluation and stabilization of airway, breathing and circulation. Urosepsis was defined as clinically evident severe infection of the urinary tract with features of SIRS [45]. In the ICU, a note of initial clinical parameters like level of consciousness using Glasgow Coma Scale (GCS), heart rate, blood pressure, respiratory rate (RR) and temperature was done. Mean arterial pressure (MAP) was calculated [678]. Initial blood and urine investigations in the form of complete blood count, random blood sugar, renal function test, arterial blood gas analysis, urine microscopy for pus cells, urine and blood culture and sensitivity tests were done. Relevant radiological investigations in the form of ultrasound abdomen and pelvis, Computed tomographic scan when indicated and chest X-ray were performed. Every attempt was made to rule out other organ sepsis.
Patients were started on antibiotics usually an empirical third generation cephalosporin or piperacillin-tazobactam. Patients were initiated on meropenem if they were treated elsewhere or if imaging suggested urinary tract obstruction with abscess or recurrent urosepsis. Patients not improving satisfactorily with conservative management were considered for surgical interventions after stabilization. Packed red blood cell transfusion was done when hemoglobin was <8 g/dL. Patients were intubated for ventilation when patients had tachypnea (RR>24/min) with hypoxia (sPO2<90% or pO2<60 mmHg), GCS<8, poor respiratory efforts or frank pulmonary edema [91011]. Inotropes were initiated when MAP was <60 mmHg and CVP was >10 cm NS. Acute Kidney Injury patients with anuria, hyperkalemia (K>6 meq/L), metabolic acidosis (pH≤7.2) or fluid overload underwent perioperative hemodialysis at the discretion of the nephrologist.
At the completion of first 24 hours after admission to the ICU, APACHE score was calculated using 12 physiological variables [45]. Points were allocated to the worst values of each variable as per protocol of APACHE-II scoring system calculation. Age and chronic health were also assigned points in the similar manner and a total APACHE score was obtained [4]. Charlson Comorbidity Index was used to assess the effect of age and comorbidities on prognosis and to classify patients [1213].
Clinical improvement was assessed using GCS and vital parameters. In postsurgical patients, who were still under the effect of anesthesia, assessment was made after the patient had recovered from anesthesia. For intubated patients, this score was calculated considering their ability to understand. Final outcome of the patient (shift out to postoperative ward or death) and total length of ICU stay were also recorded.
For the purpose of tabulation and analysis, diagnosis was coded as upper tract infection, lower tract infection, upper tract obstruction, lower tract obstruction, nonobstructive and infections. Upper tract obstruction required double J stenting or percutaneous nephrostomy as emergency procedure, also included ureterorenoscopy and lithotripsy or ureterolithotomy for ureteric calculi and percutaneous nephrolithotomy or pyelolithotomy for renal calculi. Lower urinary tract obstruction required perurethral or suprapubic cathetrisation, clot evacuation, transurethral resection of prostate or bladder tumors and prostate abscess deroofing. Nonobstructive urosepsis included nephrectomy, nephroureterectomy, radical nephrectomy or cystectomy and adrenalectomy. Infections included incision and drainage for abscesses. The highest antibiotic used was taken for analysis and antibiotic change was assessed. On urine culture analysis, staph, enterococci were grouped into a single unit. Response to surgical intervention was assessed and the modified Clavien-Dindo classification was used to grade complications [12].
Data was tabulated and statistical analysis was performed using IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA). Continuous variables were summarized as mean with standard deviation or median and interquartile range. Student t-test (2-tailed, independent) was used for comparison of metric parameters on continuous scale between the 2 groups. Categorical variables were summarized as frequencies and proportions. Chi-square and Fisher exact test were used to compare parameters on categorical scale between 2 or more groups. Binomial logistic regression analysis was used to identify independent predictors for postoperative treatment success. The area under curve (AUC) calculated by receiver operating characteristic (ROC) curve analysis was used to predict the mortality, morbidity, treatment success and need for intervention. A p-value of <0.05 was considered statistically significant.
The mean±standard deviation (SD) age was 53.52±15.65 years and 93 (52%) were male patients. Upper urinary tract infection was seen in 71 (39.9%), lower urinary tract infection in 17 (9.6%), upper urinary tract obstruction in 13 (7.3%), lower urinary tract obstruction in 54 (30.3%), nonobstructive urosepsis in 18 (10.1%) and infections in 5 patients (2.8%). Ventilator support, inotropes, hemodialysis and prolonged length of hospitalization (LOH) were seen together in 34 patients (19.1%). At least one form of morbidity (ventilator support or hemodialysis or inotropes or prolonged LOH) was observed in 166 patients (93.3%). Surgical intervention was needed in 133 (74.7%) and 38 patients (21.3%) expired (Table 1). Urine culture was positive in 131 patients (73.6%) and blood culture was positive in 47 patients (26.4%). Pyuria (>3 white blood cell/high power field) was seen in 169 patients (94.9%). Hypotension at presentation (MAP<60 mmHg) was observed in 30 patients (16.9%) and thrombocytopenia (platelet<1.5 lakh/cmm) was seen in 49 patients (27.5%). The mean±SD LOH was 13.35±9 days and ICU stay was 4.44±2.13 days.
The mean±SD APACHE II score in our study population was 26.03±7.03. APACHE II score was used to predict mortality, morbidity, need for surgical intervention and also to predict mortality despite surgical intervention using comparative statistics, univariate and multivariate analysis and ROC AUC analysis.
The mean±SD APACHE II score was 24.31±6.48 in the patients who survived and this was statistically significantly lesser than that in those who expired 32.39±5.09 (p<0.001). Among those who underwent surgery, the mean±SD APACHE II score was higher among those who expired (30.74±4.85) than those who survived after surgery (24.30±6.54) (p<0.001) (Table 2). On multivariate logistic regression, APACHE II (p<0.001), hemodialysis (p=0.032), and prolonged LOH (p=0.007) were significant factors (Table 3). ROC analysis revealed AUC of 0.825 (95% confidence interval [CI], 0.762–0.888) Based on ROC analysis, we further identified a cutoff APACHE score of 25.5 was 94.7% sensitive and 56.4% specific to predict mortality in urosepsis patients (Fig. 1A).
The mean±SD APACHE II score was 26.33±7.12 in the patients who had morbidity and this was statistically significantly lesser than that in those who did not have morbidity (22.31±4.55) (p=0.047). Morbidity included ventilator support, need for inotropes, hemodialysis, prolonged LOH and prolonged ICU stay. On multivariate analysis, APACHE II score (p=0.002), LOH (p=0.044), ICU stay (p=0.023), and male sex (p=0,045) predicted morbidity (Table 4). ROC analysis revealed AUC of 0.669 (95% CI, 0.557–0.780). Based on ROC analysis, we further identified a cutoff APACHE score of 23.5 was 64.2% sensitive and 69.2% specific to predict morbidity in urosepsis patients (Fig. 1B).
The mean±SD APACHE II score in those who underwent surgery was 25.22±6.70 and it was statistically significantly lesser than those who did not undergo surgery (28.44±7.49) (p=0.007). This could be due to the fact that patients who had not undergone surgery had significantly worse variables and were unfit for surgery. On multivariate analysis, age and APACHE II score (p<0.001), male sex (p=0.035), antibiotic change (p=0.003) and prolonged ICU stay (p=0.023) significantly predicted surgical intervention (Table 5). ROC analysis was performed for prediction of mortality in those who underwent surgery. ROC analysis revealed AUC of 0.760 (95% CI, 0.675–0.856). Based on ROC analysis, we further identified a cutoff APACHE score of 25.5 was 94.7% sensitive and 45.6% specific to predict mortality even after surgical intervention (Fig. 1C). As the mean±SD APACHE II score at 24 hours after admission increased, the modified Clavien-Dindo classification of complications was also higher (I, 24.83±5.902; II, 23.06±6.338; III, 32.50±6.364; IV, 26.52±7.077; V, 29.64±4.272; p=0.011).
APACHE II is a severity-of-disease classification system, one of several ICU scoring systems. It is applied within 24 hours of admission of a patient to an ICU and an integer score from 0–71 is computed based on several measurements. Higher scores correspond to more severe disease and a higher mortality [1415]. The first APACHE model was presented by Knaus et al. in 1981 (quoted from [4]).
Prediction of patient prognosis admitted in ICU always remains an area of great concern for physicians as well as for patient's families. The impact of this prediction bears on different aspects of patient care like selection of medical therapy, triaging, end of life care and many more [151617]. The APACHE II scoring system has been widely accepted as a measure of illness severity. It has been shown to accurately stratify risk of death in a wide range of disease states, and in different clinical settings.
Advantages of APACHE II include a single measurement, no additional investigation being needed and being well established in other system sepsis. It has been validated in several countries and has been proved to be highly reproducible. Increased scores correlated with hospital mortality (specificity >98% and sensitivity <30%). The overall correct prediction was about 80% [4]. The study makes use of easily and routinely available objective data, which could be utilized in a wide variety of hospital settings, including Accident and Emergency Departments. It may be a potentially better method of evaluating the quality of care than waiting times or reattendance rates of patients in Emergency Department. Moreover, as the worst scores were recorded in the resuscitation room before initial resuscitation actually takes place, it helps to eliminate the potential underestimation of the mortality if the worst scores were taken in the ICU after a period of aggressive resuscitation in accident and emergency resuscitation room.
We identified patients who expired, the mean APACHE II score was 32.39 and in those who died even after surgical intervention, it was 30.74. Patients with morbidity or prolonged LOH or ICU stay had a mean APACHE II score of 26.33. In patients who survived, the mean APACHE II score was 24.31. In those who survived after surgical intervention, the mean APACHE II score was 24.30. Among those who needed surgical intervention, patients who were taken up for surgery had a mean APACHE II score of 25.2 and those who were not fit for surgery it was 28.4. With these values we can reasonably predict mortality and prognosticate urosepsis using APACHE II score. When the APACHE II score at 24 hours after admission was less than 24, it predicted good prognosis, and successful surgery if indicated and patients usually survive. When the APACHE II score exceeded 30–32, there was a higher chance of mortality despite surgical intervention. Patients who had an APACHE score of 24–27 carried a high chance of morbidity, prolonged LOH and ICU stay. However they had a better outcome with surgical intervention when indicated. As APACHE II score increased, Clavien-Dindo classification of complications also increased.
APACHE III was introduced to expand and improve the prognostic estimates provided by APACHE II [18]. This system, which is only commercially available, comprises an APACHE III score and a series of predictive equations linked to diagnosis and the APACHE III database [192021]. It was not chosen for evaluation because the risk-of-death predictive equations are not available for use. The use of the APACHE II system as the severity assessment tool in Taiwan's ICUs has been taken for granted [22]. With the rapid development in the severity scoring field, further local research is imperative to justify the continuing use of this system. Tang et al. [22] reported that APACHE II is better than therapeutic intervention scoring system and the organ system failure approach in predicting mortality. The APACHE II system is quite easy to use and local professionals have become familiar with its application in intensive care [2324]. Our findings do support the argument that the application of APACHE II in ICUs is still valid despite the development of other new severity of illness measurement.
To the best of our knowledge, this is the first study to validate APACHE II score for urosepsis and it was done prospectively. We also used APACHE II score to predict mortality, morbidity, need for surgical intervention and mortality even after intervention. We included only patients with a positive bacterial urine or blood culture.
References
1. Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003; 348:138–150. PMID: 12519925.

2. Rué M, Artigas A, Alvarez M, Quintana S, Valero C. Performance of the Mortality Probability Models in assessing severity of illness during the first week in the intensive care unit. Crit Care Med. 2000; 28:2819–2824. PMID: 10966256.

3. Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993; 270:2957–2963. PMID: 8254858.

4. Markgraf R, Deutschinoff G, Pientka L, Scholten T. Comparison of acute physiology and chronic health evaluations II and III and simplified acute physiology score II: a prospective cohort study evaluating these methods to predict outcome in a German interdisciplinary intensive care unit. Crit Care Med. 2000; 28:26–33. PMID: 10667495.

5. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992; 101:1644–1655. PMID: 1303622.
6. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003; 31:1250–1256. PMID: 12682500.

7. Varpula M, Tallgren M, Saukkonen K, Voipio-Pulkki LM, Pettilä V. Hemodynamic variables related to outcome in septic shock. Intensive Care Med. 2005; 31:1066–1071. PMID: 15973520.

8. Dünser MW, Takala J, Ulmer H, Mayr VD, Luckner G, Jochberger S, et al. Arterial blood pressure during early sepsis and outcome. Intensive Care Med. 2009; 35:1225–1233. PMID: 19189077.

9. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013; 41:580–637. PMID: 23353941.

10. Orebaugh SL. Initiation of mechanical ventilation in the emergency department. Am J Emerg Med. 1996; 14:59–69. PMID: 8630160.

11. Burtin P, Bollaert PE, Feldmann L, Nace L, Lelarge P, Bauer P, et al. Prognosis of stroke patients undergoing mechanical ventilation. Intensive Care Med. 1994; 20:32–36. PMID: 8163755.

12. Dindo D, Clavien PA. What is a surgical complication? World J Surg. 2008; 32:939–941. PMID: 18414942.

13. Graefen M. The modified Clavien system: a plea for a standardized reporting system for surgical complications. Eur Urol. 2010; 57:387–389. PMID: 20022421.

14. Wong DT, Crofts SL, Gomez M, McGuire GP, Byrick RJ. Evaluation of predictive ability of APACHE II system and hospital outcome in Canadian intensive care unit patients. Crit Care Med. 1995; 23:1177–1183. PMID: 7600824.

15. Gupta R, Arora VK. Performance evaluation of APACHE II score for an Indian patient with respiratory problems. Indian J Med Res. 2004; 119:273–282. PMID: 15243165.
16. Desai S, Lakhani JD. Utility of SOFA and APACHE II score in sepsis in rural set up MICU. J Assoc Physicians India. 2013; 61:608–611. PMID: 24772695.
17. Chen FG, Koh KF, Goh MH. Validation of APACHE II score in a surgical intensive care unit. Singapore Med J. 1993; 34:322–324. PMID: 8266203.
18. Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991; 100:1619–1636. PMID: 1959406.
19. Bastos PG, Knaus WA. APACHE III study: a summary. Intensive Care World. 1991; 8:35–38. PMID: 10148199.
20. Zimmerman JE, Kramer AA. Outcome prediction in critical care: the Acute Physiology and Chronic Health Evaluation models. Curr Opin Crit Care. 2008; 14:491–497. PMID: 18787439.

21. Beck DH, Taylor BL, Millar B, Smith GB. Prediction of outcome from intensive care: a prospective cohort study comparing Acute Physiology and Chronic Health Evaluation II and III prognostic systems in a United Kingdom intensive care unit. Crit Care Med. 1997; 25:9–15. PMID: 8989170.
22. Tang CH, Yang CM, Chuang CY, Chang ML, Huang YC, Huang CF. A comparative study of clinical severity scoring systems in ICUs in Taiwan. Tzu Chi Med J. 2005; 17:239–245.
23. Rowan KM, Kerr JH, Major E, McPherson K, Short A, Vessey MP. Intensive Care Society's APACHE II study in Britain and Ireland--II: Outcome comparisons of intensive care units after adjustment for case mix by the American APACHE II method. BMJ. 1993; 307:977–981. PMID: 8241909.

24. Livingston BM, MacKirdy FN, Howie JC, Jones R, Norrie JD. Assessment of the performance of five intensive care scoring models within a large Scottish database. Crit Care Med. 2000; 28:1820–1827. PMID: 10890627.

Fig. 1
Receiver operating characteristic curves: (A) prediction of mortality by APACHE; (B) prediction of morbidity by APACHE; (C) prediction of mortality by APACHE among patients who underwent surgery. APACHE, Acute Physiology and Chronic Health Evaluation.
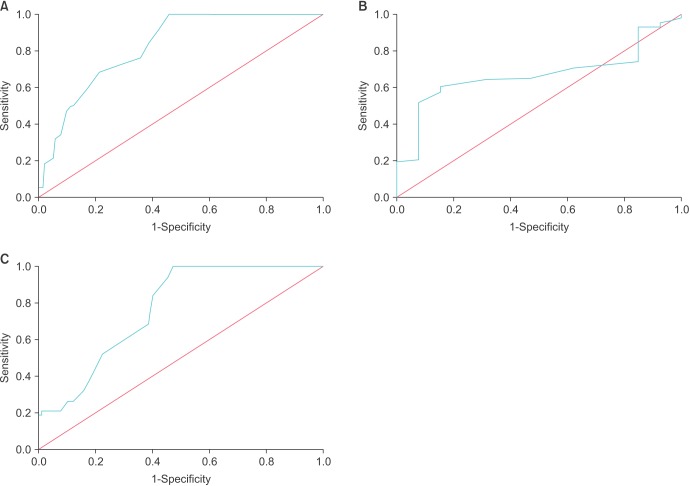
Table 1
Profile of study population
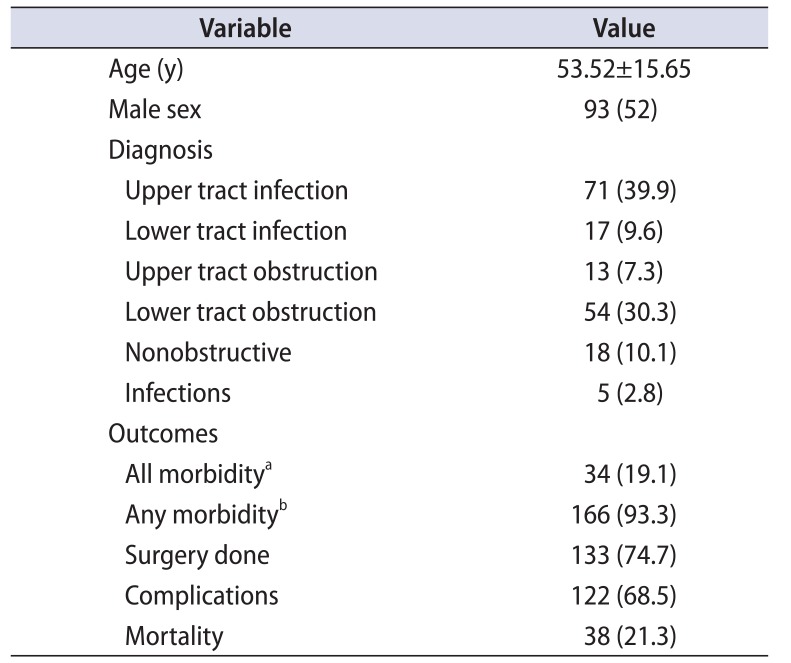
Values are presented as mean±standard deviation or number (%).
a:All morbidity: need for ventilatory support, inotropes, hemodialysis and prolonged length of hospitalization. b:Any morbidity: any one of the following - need for ventilatory support, inotropes, hemodialysis, or prolonged length of hospitalization.
Table 2
Association of APACHE II score with various outcomes
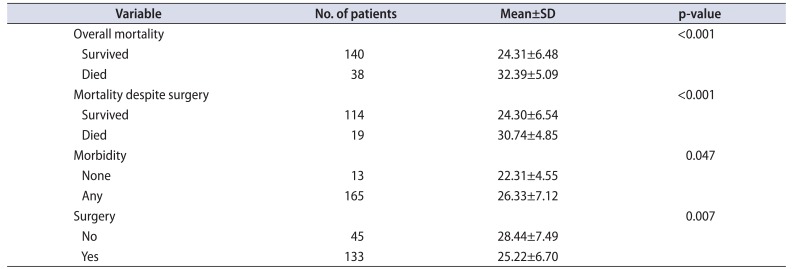
Table 3
Factors associated with mortality–multivariate analysis
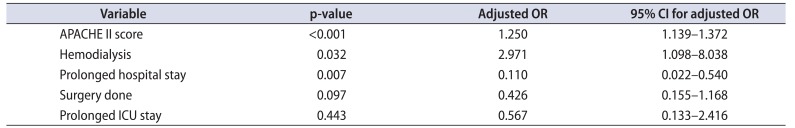
Table 4
Factors associated with morbidity–multivariate analysis
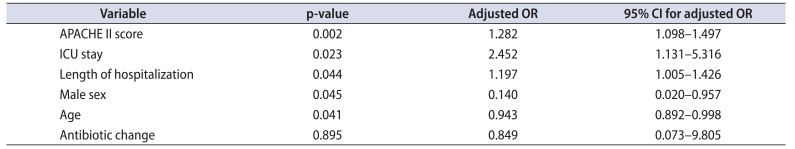
Table 5
Factors associated with need for intervention - multivariate analysis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download