Abstract
Purpose
To evaluate urination-related quality of life (QoL) in patients with an indwelling ureteral stent immediately after ureteroscopic lithotripsy (URSL) for upper urinary calculi. We compared the effects of loop-tail and pigtail ureteral stents on urination-related QoL.
Materials and Methods
Of 135 patients who underwent URSL between May 2014 and March 2015 at our hospital, we retrospectively analyzed the records of 70 patients (42 men, 28 women; median age, 63 years) in whom the stent tail was positioned inside the bladder without crossing the midline and who completed the core lower urinary tract symptoms score (CLSS) questionnaire pre- and postoperatively.
Results
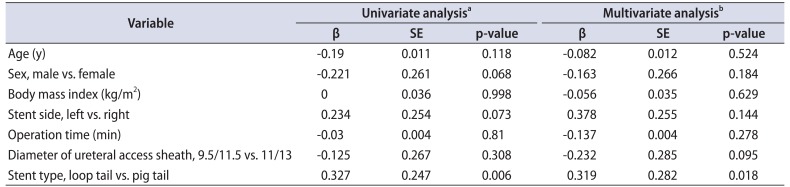
There were significant differences in incomplete emptying (p=0.048) and bladder pain (p=0.041) between patients with loop-tail versus pigtail ureteral stents after URSL. In the multivariate analysis, stent type had a stronger association with incomplete emptying (p=0.022) and bladder pain (p=0.018) than age, sex, body mass index, stent side, operation time, diameter of ureteral access sheath, and stent type.
Ureteral stents were first introduced in 1967 by Zimskind et al. [1] and have been used to relieve upper urinary tract obstruction, maintain renal function, relieve pain, and treat urinary tract infections. Ureteral stenting has also been used to aid passage of upper urinary calculi after ureteroscopic lithotripsy (URSL) and to prevent ureteral obstruction by stone fragments or hematoma. Developments in the ureteroscope and other peripheral devices have resulted in better surgical outcomes, thereby increasing the number of operations performed. Although there have been reports that ureteral stenting is unnecessary after uncomplicated URSL [2], we believe that ureteral stenting after URSL is helpful for drainage. However, ureteral stenting has been reported to diminish urination-related quality of life (QoL) in 80% of patients who undergo the procedure [3]. Some studies have reported fewer stent-related symptoms with loop-tail ureteral stents than with pigtail ureteral stents [45]. Lingeman et al. [4] reported the benefits of loop-tail stents on stent-related QoL on day 4 after URSL. We hypothesized that loop-tail stents might also decrease stent-related symptoms in the immediate post-URSL period. Therefore, in this study we aimed to evaluate the ureteral stent-related symptoms of patients with loop-tail stents in the immediate post-URSL period.
This study was approved by our Institutional Review Board (Kansai Medical University ethic committee; authorization number: 26-36). A retrospective review of prospectively collected data was performed. Between May 2014 and March 2015, 135 patients underwent URSL for upper urinary calculi at the Department of Urology and Stone Center, Kansai Medical University General Medical Center. The records of 70 of these patients in whom kidney, ureter, and bladder X-ray confirmed that the ureteral stent tail was positioned inside the bladder without crossing the midline on the day following surgery and who fully answered a questionnaire (core lower urinary tract symptoms [LUTS] score, CLSS) concerning pre- and postoperative urination-related QoL were included and analyzed. The CLSS is a questionnaire that assesses 10 urination-related symptoms with scores ranging from 0 to 3: daytime frequency, nocturia, urgency, urgency incontinence, stress incontinence, slow stream, straining, incomplete emptying, bladder pain, and urethral pain. Patients who underwent preoperative ureteral stenting or bilateral URSL, those with radiolucent stones or with performance status ≥2, and those with placement of a percutaneous nephrostomy were excluded from the present study.
One endourologist (TI) performed all procedures. All patients received general anesthesia. The URSL procedure was standardized as follows. We approached the calculi using a guide wire and semirigid ureteroscope (Olympus 8/9.8 F, Olympus Inc., Tokyo, Japan). For proximal ureteral and renal stones, we placed a ureteral access sheath under fluoroscopic guidance and fragmented the calculi with a holmium YAG laser (5–10 Hz and 0.5–1.0 J). All fragments ≥2 mm in size were extracted using a tipless basket with a flexible ureteroscope (URF-P6, Olympus Inc. or Flex-X2, Karl Storz, Tuttlingen, Germany). The ureteral access sheath used was Flexor-Cook 9.5/11.5 F (Cook Medical, Bloomington, IN, USA) or Navigator-Boston 11/13 F (Boston Scientific, Natick, MA, USA). For mid- and distal ureteral calculi, we fragmented the calculi with a holmium YAG laser and extracted all fragments ≥2 mm in size using a tipless basket with a semirigid ureteroscope without a ureteral access sheath. No cases required dilation of the ureteral orifice or ureter. At the end of the procedure the ureteral stent was left in situ. The ureteral stent was Inlay Optima (C.R. Bard Inc., Murray Hill, NJ, USA), Polaris Ultra (Boston Scientific), or Polaris Loop (Boston Scientific), depending on the date of surgery. Between May 2014 and September 2014 we used loop-tail stents; between October 2014 and March 2015 we used pigtail stents. The size of ureteral stents was fixed at 6 F. The length of loop-tail stents was 20 or 22 cm and that of pigtail stents was 22, 24, or 26 cm, according to kidney, ureter, and bladder X-ray assessment, so that the ureteral stent did not cross the bladder midline.
Urethral catheters were removed from all patients on the day following URSL. The CLSS was administered 1 day before the operation and 8 hours after urethral catheter removal on the day following URSL.
For all 70 eligible patients, background and relative changes in CLSS were evaluated. Patients were divided into 2 groups based on the type of ureteral stent used. Group 1 included patients with the Polaris Loop stent (loop-tail stent); group 2 included patients with Inlay Optima or Polaris Ultra stents (pigtail stent). We compared total number of doses of analgesic (rectal diclofenac sodium, 25 mg) used from immediately postoperatively to the day following URSL. We assessed patients' background, total number of doses of analgesic, and CLSS. Data were statistically analyzed with either the Mann-Whitney U-test or chi-square test. Furthermore, we used multivariate analysis to investigate the factors that influenced ureteral stent-related symptoms. We used multiple linear regression to assess the association between the items of significant change on the CLSS and several characteristic parameters (age, sex, body mass index, stent side, operation time, diameter of ureteral access sheath, and stent type). The IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA) was used for statistical analysis. The significance level was set at p<0.05.
Table 1 shows characteristics of the patients (n=70) and differences in patient background between groups 1 and 2. There were no postoperative complications, such as ureteral stent migration or febrile urinary tract infection, and no significant differences in patient sex, body mass index, or operation time. Patients in group 2 were significantly younger (p=0.004) and had longer stents (p<0.001).
No significant differences were observed between groups 1 and 2 for any CLSS item preoperatively.
Overall changes between pre- and postoperative CLSS are shown in Table 2. Worsening of symptoms in the immediate postoperative period resulted in significant changes in 5 CLSS items: nocturia (p=0.006), urgency (p=0.01), urgency incontinence (p=0.03), bladder pain (p=0.001), and urethral pain (p<0.001). Pre- and postoperative total scores were 6.8±4.1 and 10.8±5.5, respectively. Pre- and postoperative QoL scores were 3.3±1.5 and 4.1±1.6, respectively.
There was a significant difference between groups in the total number of doses of analgesic administered (0.2±0.5 in group 1 vs. 0.5±0.7 in group 2, p=0.02).
Table 3 shows a comparison of changes from preoperative to immediate postoperative CLSS between groups 1 and 2. There was no significant difference between groups 1 and 2 in change in mean total score (3.1±4.6 in group 1 vs. 4.5±5.1 in group 2, p=0.25) or QoL score (0.4±1.6 in group 1 vs. 1.1±1.8 in group 2, p=0.13) from pre- to immediate post-URSL for any of the 10 items. However, patients in group 1 had significantly less worsening of the scores for incomplete emptying and bladder pain than patients in group 2.
We compared immediate changes in postoperative CLSS relative to preoperative scores for patients with loop-tail versus pigtail ureteral stents. Patients with loop-tail ureteral stents had significantly less worsening of the scores for incomplete emptying and bladder pain than patients with pigtail stents. Stent type had the strongest association with incomplete emptying and bladder pain of all variables evaluated in this study.
Ureteral stents have been continuously improved and developed over the past decade, and several types of stent are currently available. Designs vary in quality of material, stent length, presence or absence of a side hole, and presence or absence of a nonpermeating X-ray marker at the end of the stent. However, impairment of QoL in post-stent implantation patients resulting from ureteral stent-related symptoms, such as incomplete emptying, bladder pain, frequency, and hematuria [46789], remains a critical concern. Ureteral stents with a loop tail on the bladder side may be used to manage such symptoms [4]. Kawahara et al. [5] reported in a prospective study that replacing a pigtail stent with a loop-tail stent alleviated symptoms of incomplete emptying, frequency, intermittency, urgency, weak stream, straining, incontinence, and urethral and flank pain in 25 patients. However, ureteral stent-related symptoms were not evaluated with a validated questionnaire and the number of cases was small in that study. Lingeman et al. [4] prospectively compared pigtail and loop-tail ureteral stents in 236 post-URSL patients using the ureteric stent symptoms questionnaire (USSQ). They found that the pain score on day 4 after placement improved with loop-tail stents and that patients with loop-tail ureteral stents had lower pain medication usage on day 1 after placement. Our study confirmed that bladder pain was less severe with looptail stents than with pigtail stents on day 1 after placement. Given these reports indicating the advantages of loop-tail stents over pigtail stents regarding ureteral stent-related symptoms, loop-tail stents should be the preferred option for patients with an indwelling ureteral stent. Although the USSQ is useful for the evaluation of ureteral stent-related symptoms and QoL after ureteral stent placement [101112], there is no validated Japanese version. Therefore, in the present study we evaluated urination-related QoL with the CLSS.
The CLSS questionnaire assesses 10 urination-related symptoms, each scored on a scale from 0 to 3 [13]. This simple questionnaire can evaluate the disease-specific patterns of LUTS and is applicable to both men and women with a wide variety of urinary impairments [131415]. Fujimura et al. [14] compared LUTS assessment with the International Prostate Symptom Score (IPSS) versus the CLSS and reported that the CLSS questionnaire is more comprehensive than the IPSS questionnaire for symptom assessment. Therefore, the CLSS is widely used to assess LUTS. Because the IPSS was originally designed to assess the symptoms of benign prostatic hyperplasia, we used the CLSS to assess LUTS in the present study.
In the present study, patients with a loop-tail stent experienced less worsening of bladder pain in the immediate post-URSL period than patients with a pigtail stent. However, patients with a pigtail stent were younger than those with a loop-tail stent. The reported correlations between ureteral stent-related symptoms and age have been inconsistent [1617]. Kuehhas et al. [16] used a visual analog scale to assess the pain of 124 patients with an indwelling ureteral stent. They reported that age did not correlate with stent-related pain scores. Olvera-Posada et al. [17] used the USSQ to assess ureteral stent-related symptoms in 44 patients and reported no significant difference in symptoms according to age. In the present study we were unable to find a correlation (analyzed with Spearman rank correlation; data not shown) between age and CLSS score. Therefore, the age difference between the groups may have had at most only a slight effect on pain scores. Giannarini et al. [18] reported a significant association between USSQ score and sex according to multivariate analysis. However, multiple linear regression analysis in the present study did not reveal a significant association between incomplete emptying and patient sex (p=0.066). Stent type had the strongest association with incomplete emptying and bladder pain in this study.
Few studies have evaluated the relationship between ureteral stent-related symptoms and stent positioning. Abt et al. [19] reported that intravesical stent position did not significantly influence stent-related symptoms. However, that study was retrospective and the day on which patients assessed their ureteral stent-related symptoms was not uniform (ranging from day 8 to 94 after stenting). Studies have reported that excessively long ureteral stents cause the placement to cross the bladder midline, worsening urinary symptoms [18192021]. Therefore, we considered the ureteral stent position important when evaluating ureteral stent-related symptoms. In this study the position of ureteral stents was standardized so as not to cross the bladder midline to prevent migration of the ureteral stent. Kawahara et al. [5] reported previously that the length of loop-tail ureteral stents was about 2 cm shorter than pigtail stents. This finding explains the stent length difference between our 2 treatment groups. However, the difference in stent length did not affect CLSS scores between the groups.
Our results demonstrate that urination status worsened after URSL. Patients specifically reported bladder pain and urethral pain, symptoms that can cause patients severe psychological trauma and prevent them from consenting to subsequent surgery if urinary calculi recur. Such pain, therefore, should be managed and alleviated to the greatest possible extent. Urethral pain and bladder pain may be caused mainly by intraoperative manipulation of the rigid ureteroscope and postoperative placement of the urethral catheter and stent. Urethral pain is likely attributable to manipulation of a rigid ureteroscope and urethral catheter, and bladder pain predominantly to manipulation of a rigid ureteroscope and urethral catheter or stent. Therefore, great care is taken at our center to have a single endourologist manipulate the ureteroscope as gently as possible and to carefully follow standardized surgical practice. In addition, the urethral catheter and stent are removed as soon as possible after the URSL procedure [22]. Because ureteral stents may exacerbate urinary symptoms if placed so that they can cross the bladder midline [18192021], we place the stent specifically to avoid crossing the midline.
This study has some limitations. First, it was retrospective and nonrandomized. The choice of ureteral stent was entirely up to the operator. Second, urination-related QoL was evaluated on the day following the operation in this study, although urination-related QoL is generally evaluated 1 to 2 weeks after ureteral stent placement [23242526]. However, we found a significant difference in ureteral stent-related symptoms in the immediate post-URSL period between patients with loop-tail versus pigtail stents, and we consider these symptoms also important. Third, this study used the CLSS, although the USSQ is globally considered the gold standard [12] for the evaluation of ureteral stent-related symptoms. Future studies should re-evaluate our findings using the USSQ. Fourth, we used 2 types of pigtail stent, the Inlay and Polaris. The difference in the type of pigtail stent might have affected ureteral stent-related symptoms. However, we compared CLSS scores in patients with the Polaris versus Inlay stents and found no significant differences in total score, QoL score, or in any of the 10 items (data not shown). Furthermore, Davenport et al. [27] found no significant difference in ureteral stent-related symptoms in patients with Polaris versus Inlay stents in a prospective randomized controlled study. Therefore, we consider it unlikely that the type of pigtail stent influenced the comparison of pigtail and loop-tail stents in this study.
The present study revealed that with standardized positioning, the loop-tail stent was associated with less worsening of incomplete emptying and bladder pain in the immediate post-URSL period. The URSL procedure significantly affected urinary symptoms. However, the loop-tail ureteral stent is a potentially beneficial option to minimize ureteral stent-related symptoms.
References
1. Zimskind PD, Fetter TR, Wilkerson JL. Clinical use of long-term indwelling silicone rubber ureteral splints inserted cystoscopically. J Urol. 1967; 97:840–844. PMID: 6025928.

2. Başeskioğlu B, Sofikerim M, Demirtaş A, Yenilmez A, Kaya C, Can C. Is ureteral stenting really necessary after ureteroscopic lithotripsy with balloon dilatation of ureteral orifice? A multi-institutional randomized controlled study. World J Urol. 2011; 29:731–736. PMID: 21590466.

3. Joshi HB, Okeke A, Newns N, Keeley FX Jr, Timoney AG. Characterization of urinary symptoms in patients with ureteral stents. Urology. 2002; 59:511–516. PMID: 11927301.

4. Lingeman JE, Preminger GM, Goldfischer ER, Krambeck AE. Comfort Study Team. Assessing the impact of ureteral stent design on patient comfort. J Urol. 2009; 181:2581–2587. PMID: 19375088.

5. Kawahara T, Ito H, Terao H, Ogawa T, Uemura H, Kubota Y, et al. Changing to a loop-type ureteral stent decreases patients' stent-related symptoms. Urol Res. 2012; 40:763–767. PMID: 22899382.

6. Damiano R, Autorino R, De Sio M, Cantiello F, Quarto G, Perdonà S, et al. Does the size of ureteral stent impact urinary symptoms and quality of life? A prospective randomized study. Eur Urol. 2005; 48:673–678. PMID: 16039775.

7. Hendlin K, Vedula K, Horn C, Monga M. In vitro evaluation of ureteral stent compression. Urology. 2006; 67:679–682. PMID: 16600353.

8. Joshi HB, Chitale SV, Nagarajan M, Irving SO, Browning AJ, Biyani CS, et al. A prospective randomized single-blind comparison of ureteral stents composed of firm and soft polymer. J Urol. 2005; 174:2303–2306. PMID: 16280829.

9. Pedro RN, Hendlin K, Kriedberg C, Monga M. Wire-based ureteral stents: impact on tensile strength and compression. Urology. 2007; 70:1057–1059. PMID: 18158013.

10. Joshi HB, Newns N, Stainthorpe A, MacDonagh RP, Keeley FX Jr, Timoney AG. Ureteral stent symptom questionnaire: development and validation of a multidimensional quality of life measure. J Urol. 2003; 169:1060–1064. PMID: 12576846.

11. Joshi HB, Stainthorpe A, Keeley FX Jr, MacDonagh R, Timoney AG. Indwelling ureteral stents: evaluation of quality of life to aid outcome analysis. J Endourol. 2001; 15:151–154. PMID: 11325084.
12. Miyaoka R, Monga M. Ureteral stent discomfort: Etiology and management. Indian J Urol. 2009; 25:455–460. PMID: 19955667.

13. Homma Y, Yoshida M, Yamanishi T, Gotoh M. Core Lower Urinary Tract Symptom score (CLSS) questionnaire: a reliable tool in the overall assessment of lower urinary tract symptoms. Int J Urol. 2008; 15:816–820. PMID: 18657204.

14. Fujimura T, Kume H, Nishimatsu H, Sugihara T, Nomiya A, Tsurumaki Y, et al. Assessment of lower urinary tract symptoms in men by international prostate symptom score and core lower urinary tract symptom score. BJU Int. 2012; 109:1512–1516. PMID: 21883834.

15. Fujimura T, Kume H, Tsurumaki Y, Yoshimura Y, Hosoda C, Suzuki M, et al. Core lower urinary tract symptom score (CLSS) for the assessment of female lower urinary tract symptoms: a comparative study. Int J Urol. 2011; 18:778–784. PMID: 21951201.

16. Kuehhas FE, Miernik A, Sharma V, Sevcenco S, Javadli E, Herwig R, et al. A prospective evaluation of pain associated with stone passage, stents, and stent removal using a visual analog scale. Urology. 2013; 82:521–525. PMID: 23768523.

17. Olvera-Posada D, Suárez-Santos M, Castillejos-Molina R, Gabilondo-Navarro F, Méndez-Probst CE. Validation of the Spanish version of Ureteral Stent Symptom Questionnaire: prevalence of symptoms in a tertiary care center in Mexico. J Endourol. 2014; 28:377–382. PMID: 24112085.

18. Giannarini G, Keeley FX Jr, Valent F, Manassero F, Mogorovich A, Autorino R, et al. Predictors of morbidity in patients with indwelling ureteric stents: results of a prospective study using the validated Ureteric Stent Symptoms Questionnaire. BJU Int. 2011; 107:648–654. PMID: 20590539.

19. Abt D, Mordasini L, Warzinek E, Schmid HP, Haile SR, Engeler DS, et al. Is intravesical stent position a predictor of associated morbidity? Korean J Urol. 2015; 56:370–378. PMID: 25964838.

20. Rane A, Saleemi A, Cahill D, Sriprasad S, Shrotri N, Tiptaft R. Have stent-related symptoms anything to do with placement technique? J Endourol. 2001; 15:741–745. PMID: 11697408.

21. Ho CH, Chen SC, Chung SD, Lee YJ, Chen J, Yu HJ, et al. Determining the appropriate length of a double-pigtail ureteral stent by both stent configurations and related symptoms. J Endourol. 2008; 22:1427–1431. PMID: 18613783.

22. Tang L, Gao X, Xu B, Hou J, Zhang Z, Xu C, et al. Placement of ureteral stent after uncomplicated ureteroscopy: do we really need it? Urology. 2011; 78:1248–1256. PMID: 21762964.

23. Park HK, Paick SH, Kim HG, Lho YS, Bae S. The impact of ureteral stent type on patient symptoms as determined by the ureteral stent symptom questionnaire: a prospective, randomized, controlled study. J Endourol. 2015; 29:367–371. PMID: 25153249.

24. Sanguedolce F, Millán-Rodriguez F, Santillana-Altimira JM, Fantova-Alonso A, Sánchez-Martín FM, Angerri-Feu O, et al. The Spanish linguistic validation of the ureteral stent symptom questionnaire. J Endourol. 2014; 28:237–242. PMID: 24032342.

25. Dellis AE, Keeley FX Jr, Manolas V, Skolarikos AA. Role of α-blockers in the treatment of stent-related symptoms: a prospective randomized control study. Urology. 2014; 83:56–61. PMID: 24210570.

26. Calvert RC, Wong KY, Chitale SV, Irving SO, Nagarajan M, Biyani CS, et al. Multi-length or 24 cm ureteric stent? A multicentre randomised comparison of stent-related symptoms using a validated questionnaire. BJU Int. 2013; 111:1099–1104. PMID: 22882647.
27. Davenport K, Kumar V, Collins J, Melotti R, Timoney AG, Keeley FX Jr. New ureteral stent design does not improve patient quality of life: a randomized, controlled trial. J Urol. 2011; 185:175–178. PMID: 21074809.

Table 1
Characteristics of all patients and of patients according to treatment group
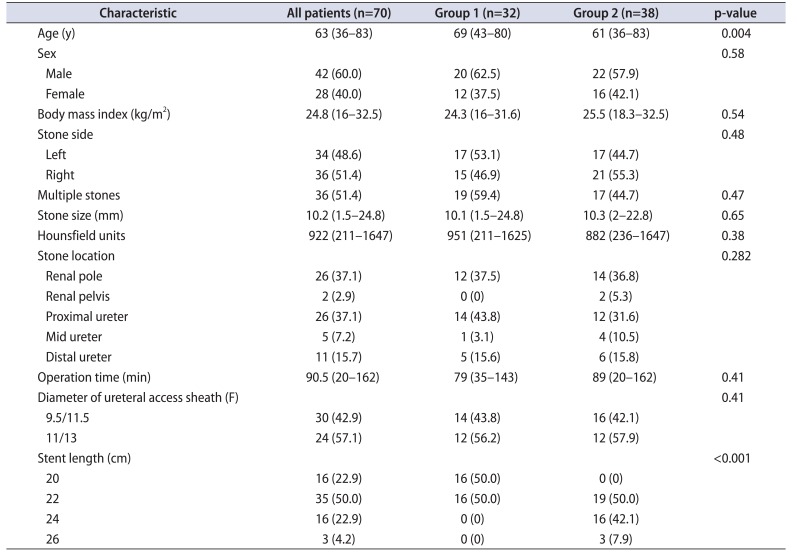
Table 2
Pre- and postoperative CLSS scores for all patients
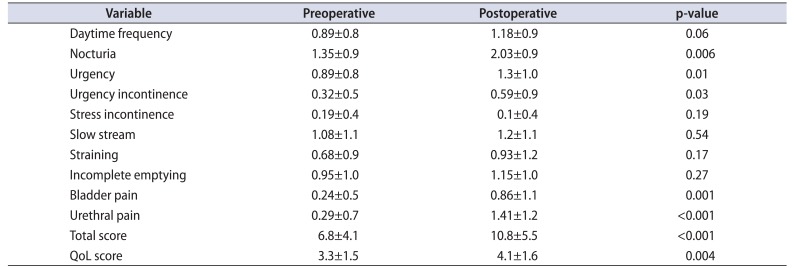
Table 3
Changes in CLSS score from preoperative score to postoperative score, according to treatment group
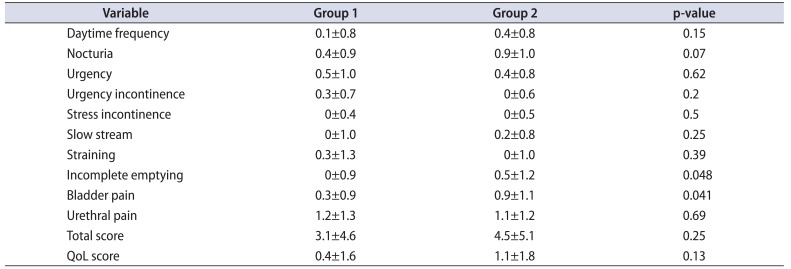
Table 4
Univariate and multivariate analysis assessing association between incomplete emptying and characteristic parameters
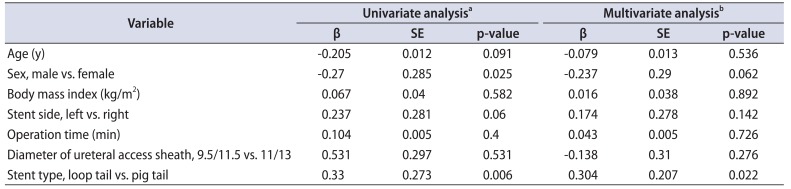
Table 5
Univariate and multivariate analysis assessing association between bladder pain and characteristic parameters




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download