Abstract
Purpose
To assess the impact of proteinuria on postoperative renal function after open partial nephrectomy (OPN) in patients with a solitary kidney and analyze predictive factors for developing chronic kidney disease (CKD) stage IV or higher.
Materials and Methods
Patients with a solitary kidney who underwent OPN at Tokyo Women's Medical University Hospital between 1986 and 2016 were the subjects of this study. The patients were divided into 2 groups according to the presence of preoperative proteinuria. The development rate of stage IV CKD or higher was analyzed by the Kaplan-Meier method to compare the postoperative renal function of the 2 groups. Multivariate regression analysis was used to demonstrate predictive factors for postoperative CKD progression.
Results
A total of 96 patients, including 73 without proteinuria and 23 with proteinuria, were included in this study. Patients with proteinuria were more likely to be men (95.6% vs. 64.3%, p<0.01), had a higher body mass index (25.7 kg/m2 vs. 23.5 kg/m2, p<0.01), and had a higher incidence of hypertension (69.5% vs. 39.7%, p=0.01). Patients with proteinuria had a higher probability of developing stage IV CKD or higher (p=0.0002). Lower preoperative eGFR (p<0.0001) and positive proteinuria (p=0.04) were independent predictors for CKD stage progression on multivariate analysis.
Conclusions
Preoperative proteinuria and eGFR were independent predictors for developing stage IV CKD or higher after OPN. Meanwhile, surgical factors including ischaemia time and tumor size had no significant effect. This suggests that assessment of preoperative CKD stage could help stratify patients according to their risk of renal function exacerbation.
Nephron sparing surgery (NSS) provides superior renal functional outcomes compared to radical nephrectomy without compromising oncologic outcomes and is strongly indicated for patients with a solitary kidney. Renal function preservation for the solitary kidney helps prevent the development of end-stage renal disease (ESRD) requiring hemodialysis. Patients on hemodialysis have a higher risk of cardiovascular events and lower survival rates compared to patients who do not receive hemodialysis. Thus, efforts to delay the start of hemodialysis are clinically important to improve the quality of life and patient survival [123]. An international work group has classified stages of chronic kidney disease (CKD), which are based on the cause, glomerular filtration rate (GFR), and albuminuria [4]. Preoperative proteinuria as well as GFR reportedly impact overall survival of patients undergoing nephrectomy [5]. CKD is more likely to progress in patients who have CKD due to medical reasons compared to those who have the same degree of CKD caused by NSS [6]. These reports suggest the impact of preexisting medical impairment of the kidney on postoperative renal functional outcomes. To the best of our knowledge, there are few articles concerning the impact of proteinuria on renal functional outcomes in patients with a solitary kidney. This patient cohort evaluates a unique aspect of NSS, in which the contralateral kidney does not compensate for the ipsilateral renal functional decline. Patients with a solitary kidney are vulnerable to ESRD. For patients with stage CKD III or lower, we concentrate on the management of risk factors such as hypertension (HT) and diabetes mellitus (DM). However, once patients progress to stage IV CKD or higher they have to start additional treatment such as volume control, renal anemia, electrolyte and bone mineral modification, and hemodialysis; therefore, determining the risk factors for renal functional decline to stage IV CKD or higher is clinically important. The aim of our study was to investigate the impact of proteinuria on renal functional outcomes after open partial nephrectomy (OPN) in patients with a solitary kidney.
Institutional Review Board approval at Tokyo Women's Medical University was obtained to retrospectively analyze patient data (approval number: 4012). The IRB waived written Informed Consent from the subjects. We evaluated 865 patients who underwent OPN for renal tumors at a single institution between 1986 and 2016. Of these, the patients whose follow-up time and outcome data were available from medical records were included in the study. A total of 102 patients had an anatomically or functionally solitary kidney. Six patients who underwent ex vivo partial nephrectomy and autotransplantation were excluded. In total, 96 patients were eligible for the study. The following variables were considered for each patient: age, sex, American Society of Anesthesiologists (ASA) physical status classification, body mass index, presence of HT and DM, urine dipstick test, tumor diameter, radius, exophytic/endophytic, nearness of tumor to the collecting system or sinus, anterior/posterior, location relative to polar lines (RENAL) nephrometry score, preoperative and postoperative renal function, operating time, estimated blood loss, cold ischemia time (CIT), and incidence of complications. Renal function was assessed using the estimated glomerular filtration rate (eGFR) within one month before surgery and 3, 6, and 12 months after surgery. eGFR was calculated using the Modification of Diet in Renal Disease 2 equation modified for Japanese patients as outlined by The Japanese Society of Nephrology (eGFR=1.94×serum creatinine mg/dL1.094×age×[0.739 if female]) [7]. Preoperative proteinuria was grouped as 1+, 2+, and 3+ by using the dipstick method for urinary protein.
A retroperitoneal approach was made with a flank incision from the tip of the eleventh rib to the edge of the abdominal rectus muscle, exposing the kidney and renal hilum. The renal artery and vein were clamped en bloc at the renal hilum, and then ice slush was placed around the kidney. After 5 minutes of cooling, the tumor was resected with 2–5 mm of the parenchymal margin; the transected vessels were ligated with 4-0 absorbable suture, and the opened collecting system was repaired with 4-0 absorbable suture. The renal parenchyma was coagulated with monopolar coagulation (SOFT COAG, VIO 300D; ERBE Elektromedizin, Tubingen, Germany), avoiding the renal hilum; a TachoSil tissue-sealing sheet (CSL Behring Japan, Tokyo, Japan) was placed in the resected bed and manually compressed for five to 10 minutes after unclamping the renal hilum. Parenchymal renorrhaphy was added with blind 1-0 or 2-0 absorbable interrupted sutures (1- to 2-cm pitch and width) depending on the surgeon's preference. To simplify the procedure, we do not routinely use intraoperative normal saline or indigotindisulfonate injection through a ureteral catheter. For postoperative management, strict monitoring of body fluid balance between urine output, perspiration, and intravenous fluid was performed. If physical or laboratory findings of acute kidney injury appeared, serum creatinine continued to rise, or oliguria persisted, then temporary dialysis was performed. If there was no urine leakage or bleeding, the drain was removed and the patient was discharged. Urine leakage was defined as persistent drain output more than 48 hours after partial nephrectomy with chemical analysis consistent with urine [8].
We compared patient and tumor characteristics and perioperative outcomes of the 2 cohorts with and without preoperative proteinuria. The Mann-Whitney U-test was used for continuous variables. The chi-square test was used to estimate unordered categorical variables, and the Mann-Whitney U-test was used to adjust for ordinal categorical variables. We used the Cox proportional hazard model to investigate the predictive factors for postoperative eGFR decline <30 mL/min/1.73 m2, considering covariates such as age, tumor, size, preoperative eGFR, and CIT. Kaplan-Meier survival curves were generated comparing the 2 groups with or without proteinuria with bands of 95% confidence intervals (CIs). All statistical analyses were done with JMP 11 (SAS Institute, Cary, NC, USA). p<0.05 was considered statistically significant.
For all 96 patients who underwent OPN with a solitary kidney with eGFR≥30 mL/min/1.73 m2, a urine dipstick test was performed within 4 weeks before surgery. Overall, 23 patients were positive for proteinuria on the dipstick test. Of the patients with proteinuria, 13 patients had 1+, 9 patients had 2+, and 1 patient had 3+ proteinuria. The cause of proteinuria was renal sclerosis in 12 patients, glomerulonephritis in 5 patients, and DM nephropathy in 3 patients. In the present analysis, we included all patients with proteinuria into a single subset. Table 1 lists preoperative patient characteristics, comparing patients and without proteinuria. In the cohort of patients positive for proteinuria, most of the patients were male, body mass index was significantly higher (p<0.01), and the number of patients with HT was significantly higher (p=0.01). The number of patients who were on angiotensin converting enzyme inhibitors or angiotensin receptor blockers was not statistically different when comparing those with and without proteinuria. The mean age, ASA physical status classification, and preoperative eGFR did not differ significantly between patients with and without proteinuria. Tumor size and tumor location did not show any statistically significant difference.
Table 2 shows the surgical outcomes of these patients. Operative time in the group with proteinuria tended to be longer although the difference did not reach statistical significance. There was no significant difference in estimated blood loss, CIT, or length of postoperative hospital stay between the 2 groups. As a postoperative complication, urine leakage occurred in 2 patients, and an asymptomatic renal artery pseudoaneurysm occurred in 1 patient, which was Clavien-Dindo grade ≥III. Urine leakage was treated with ureteral stent placement, and asymptomatic renal artery pseudoaneurysm was treated with prophylactic transarterial embolism. Temporary hemodialysis due to acute renal failure was required in three patients who did not have proteinuria. No patients needed chronic hemodialysis during the median follow-up time of 42 months (interquartile range, 21–70 months). Postoperative relative decline in eGFR after surgery was not significantly different between the 2 groups and tended to be greater in the group of patients positive for proteinuria at 12 months (-24% vs. -18%, p=0.25), although this did not reach statistical significance.
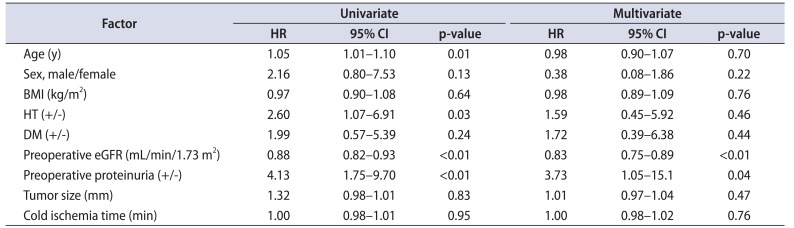
Of the 96 patients, new onset stage IV CKD or higher was detected in 22 patients during the median follow-up time. We performed Cox proportional hazard analysis to identify individual variables associated with postoperative renal function decline (new onset stage IV CKD or higher). On univariate analysis, we identified 4 factors associated with progression to stage IV CKD or higher, which were age, presence of HT, preoperative eGFR, and preoperative proteinuria. On multivariate analysis, lower preoperative eGFR (odds ratio [OR], 0.83 per mL/min/1.73 m2; 95% CI, 0.75–0.89), and presence of preoperative proteinuria (OR 3.73, 95% CI, 1.05–15.1) remained the risk factors associated with the progression to CKD stage IV or higher (Table 3). Subsequently, the Kaplan-Meier method (Fig. 1) showed higher risk of developing stage IV CKD or higher in patients positive for proteinuria than in those without proteinuria (p=0.0002); the probability of eGFR being ≥30 mL/min/1.73 m2 after 24 months was 57% vs. 87%.
The Kidney Disease: Improving Global Outcomes (KDIGO) Chronic Kidney Disease guideline classification is based on cause, GFR, and proteinuria [9]. In the present study, we analysed the impact of proteinuria on renal functional decline after OPN in a solitary kidney. We focused on patients with a solitary kidney to more precisely evaluate the impact of proteinuria. Patients with a solitary kidney are vulnerable to ESRD, and chronic haemodialysis ruins the quality of life. Therefore, it is clinically beneficial to identify the risk factors for renal functional decline in this patient cohort. From our data set, the presence of proteinuria and reduced eGFR had a statistically significant association with progression to stage IV CKD or higher after surgery. Moreover, the presence of preoperative proteinuria showed a significantly negative impact on new onset stage IV CKD or higher.
Recently, several articles have been published concerning the negative impact of proteinuria on postoperative renal function. Tourojman et al. [5] reported that preoperative proteinuria as well as preoperative GFR is a significant predictor of overall survival, independent of cancer stage. In their study, 362 and 538 patients received partial and radical nephrectomy, respectively. The decreased overall survival was presumably due to cardiovascular disease predisposed by CKD stage progression, although the specific course of GFR decline after surgery was not provided. They concluded that precise staging of CKD with GFR and the extent of proteinuria should be considered when assessing prognosis and treatment strategy [5]. Currently, the number of cases treated with robot-assisted partial nephrectomy (RAPN) is increasing, and the impact of proteinuria on renal function has also been studied among patients treated with RAPN. Krane et al. [10] reported 269 cases of RAPN with preoperative eGFR >60, out of which 57 patients had proteinuria. During the median follow-up period of 16 months, preoperative proteinuria increased the risk of progression to stage III CKD (OR, 2.3; 95% CI, 1.03–4.95). The presence of preoperative proteinuria can be a reliable factor when deciding upon treatment options, including radical nephrectomy and partial nephrectomy (OPN, RAPN).
Our study cohort of a solitary kidney provides a unique perspective on renal functional outcome after partial nephrectomy because of the lack of a normally compensating contralateral kidney. We investigated the length of time patients had a solitary kidney to evaluate the effect of compensatory hyperplasia after contralateral nephrectomy. Most of the periods were long: 5 to 10 years in 17 cases, more than 10 years in 44 cases, and unknown in 28 cases. Furthermore, there was no significant difference between the groups with and without proteinuria. Therefore, we assumed that the preoperative solitary kidney status was likely stable and not affected by compensatory hyperplasia. A solitary kidney will be affected by ischaemia as well as parenchymal volume loss following acute renal failure, which may necessitate temporary hemodialysis. We encountered three patients with acute renal failure that required emergent hemodialysis, but none of these three patients had preoperative proteinuria. Thus, the presence of proteinuria is unlikely to be associated with acute kidney injury. All of them were able to get off hemodialysis, and none needed chronic hemodialysis during our study period. Because of the imperative indication for tumors in a solitary kidney, some cases are challenging in terms of tumor size, location, and repeat surgery. In fact, among the patients who underwent surgery in our institute, the mean operative time 231 minutes vs. 210 minutes, p<0.01) and estimated blood loss (330 mL vs. 206 mL, p<0.01) in the patients with a solitary kidney was greater, and cold ischaemia time was almost same (42 minutes vs. 41 minutes, p=0.77), although mean tumour size was smaller (32 mm vs. 38 mm, p<0.01), compared to the patients with a normal contralateral kidney. The surgical procedure for OPN has already been standardised as described above, and the surgeon's preference is limited. The surgical procedure including parenchymal volume loss and CIT is considered a major predictive factor of renal functional decline after partial nephrectomy [11], but our results did not reach statistical significance. Thus, their influence on postoperative renal function seems minimal even though cold ischemia was minimized as shown in our data. Meanwhile, intrinsic patient factors including age, presence of HT, preoperative eGFR, and the presence of proteinuria showed statistical significance. These outcomes may imply a greater significance of intrinsic factors compared to factors associated with the surgical procedure for patients with a solitary kidney who are at a higher risk of ESRD. We assume that renal functional outcome may be influenced by intrinsic factors for patients with a solitary kidney. All the risk factors remaining in our results were known risk factors for CKD progression, indicating that patients who have a risk of CKD progression are likely to progress whether or not they undergo partial nephrectomy. Meanwhile, patients who do not have CKD risk factors are less affected by partial nephrectomy [9]. In this regard, surgeons have to pay greater attention to the perioperative management of comorbidities that are risk factors. This information can be used to predict the timing of hemodialysis after surgery, and both physicians and patients can prepare for the possible consequences.
As the prevalence of minimally invasive surgery including RAPN is increasing, the number of renal tumors in a solitary kidney treated with RAPN is also increasing. For this technique, precise tumor resection for parenchymal volume preservation and limited warm ischemia time, including early unclamping, partial clamping, or zero ischemia, is indispensable [121314]. At our institution, we have limited experience with RAPN for tumors in a solitary kidney, and its postoperative renal functional outcome was clinically acceptable. It remains an issue of interest whether proteinuria affects the postoperative renal function in a patient with a solitary kidney treated with RAPN.
In the present study, we did not assess the extent of proteinuria or the cause of proteinuria because of the limited sample size. We routinely examined urine dipstick tests within a month before surgery but did not quantify the amount of proteinuria, such as a 24-hour urine collection following a positive detection. As described in the KDIGO CKD guideline, the cause as well as proteinuria and GFR is an important factor for classifying the severity of CKD. Further investigation, including quantification of proteinuria and classification of cause, may clarify the impact of proteinuria on renal functional outcome in patients with a solitary kidney.
This study has several limitations, including its retrospective nature and collection of data from a single tertiary centre. The initial series in our study period lacked some tumor characteristics data, including RENAL-NS. In addition, in the present study we included all patients with proteinuria as a single subset and did not quantify the amount of urine protein. We did not analyze the association of the cause of CKD because of the limited sample size. Although the severity of DM nephropathy is linked to the extent of proteinuria, our dataset had a small number of patients with DM nephropathy [15]. However, the size of the study population was not small compared to previous studies concerning solitary kidney, and it could show the statistical significance of proteinuria adjusting covariates.
In conclusion, we analysed the risk factors of CKD progression in the unique cohort of patients with a solitary kidney and provided evidence that proteinuria may have a negative impact on CKD progression. Patients with a solitary kidney are vulnerable to ESRD, and evaluation of CKD risk factors including routine examination of proteinuria before surgery is important for decision making for both surgeons and patients.
References
1. Weight CJ, Lieser G, Larson BT, Gao T, Lane BR, Campbell SC, et al. Partial nephrectomy is associated with improved overall survival compared to radical nephrectomy in patients with unanticipated benign renal tumours. Eur Urol. 2010; 58:293–298. PMID: 20546991.

2. Tan HJ, Norton EC, Ye Z, Hafez KS, Gore JL, Miller DC. Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA. 2012; 307:1629–1635. PMID: 22511691.

3. Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors: is there a difference in mortality and cardiovascular outcomes? J Urol. 2009; 181:55–61. PMID: 19012918.
4. Stevens PE, Levin A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013; 158:825–830. PMID: 23732715.

5. Tourojman M, Kirmiz S, Boelkins B, Noyes SL, Davis AT, O'Donnell K, et al. Impact of reduced glomerular filtration rate and proteinuria on overall survival of patients with renal cancer. J Urol. 2016; 195:588–593. PMID: 26433140.

6. Lane BR, Demirjian S, Derweesh IH, Riedinger CB, Fergany AF, Campbell SC. Is all chronic kidney disease created equal? Curr Opin Urol. 2014; 24:127–134. PMID: 24451089.

7. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009; 53:982–992. PMID: 19339088.

8. Meeks JJ, Zhao LC, Navai N, Perry KT Jr, Nadler RB, Smith ND. Risk factors and management of urine leaks after partial nephrectomy. J Urol. 2008; 180:2375–2378. PMID: 18930268.

9. Andrassy KM. Comments on ‘KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease’. Kidney Int. 2013; 84:622–623.

10. Krane LS, Heavner MG, Peyton C, Rague JT, Hemal AK. Association of urine dipstick proteinuria and postoperative renal function following robotic partial nephrectomy. J Endourol. 2016; 30:532–536. PMID: 26714737.

11. Lane BR, Babineau DC, Poggio ED, Weight CJ, Larson BT, Gill IS, et al. Factors predicting renal functional outcome after partial nephrectomy. J Urol. 2008; 180:2363–2368. PMID: 18930264.

12. Satkunasivam R, Tsai S, Syan S, Bernhard JC, de Castro Abreu AL, Chopra S, et al. Robotic unclamped “minimal-margin” partial nephrectomy: ongoing refinement of the anatomic zero-ischemia concept. Eur Urol. 2015; 68:705–712. PMID: 26071789.

13. Peyronnet B, Baumert H, Mathieu R, Masson-Lecomte A, Grassano Y, Roumiguié M, et al. Early unclamping technique during robot-assisted laparoscopic partial nephrectomy can minimise warm ischaemia without increasing morbidity. BJU Int. 2014; 114:741–747. PMID: 24690155.

14. Simone G, Gill IS, Mottrie A, Kutikov A, Patard JJ, Alcaraz A, et al. Indications, techniques, outcomes, and limitations for minimally ischemic and off-clamp partial nephrectomy: a systematic review of the literature. Eur Urol. 2015; 68:632–640. PMID: 25922273.

15. Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med. 1999; 341:1127–1133. PMID: 10511612.

Fig. 1
New onset of severe chronic kidney disease (stage IV or higher) after partial nephrectomy according to the presence of preoperative proteinuria. In the Kaplan-Meier curves, patients with proteinuria had a higher risk of developing stage IV or higher chronic kidney disease than those without proteinuria (p=0.0002); the probability of an estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73 m2 after 24 months was 57% vs. 87%.

Table 1
Characteristics of patients undergoing open partial nephrectomy
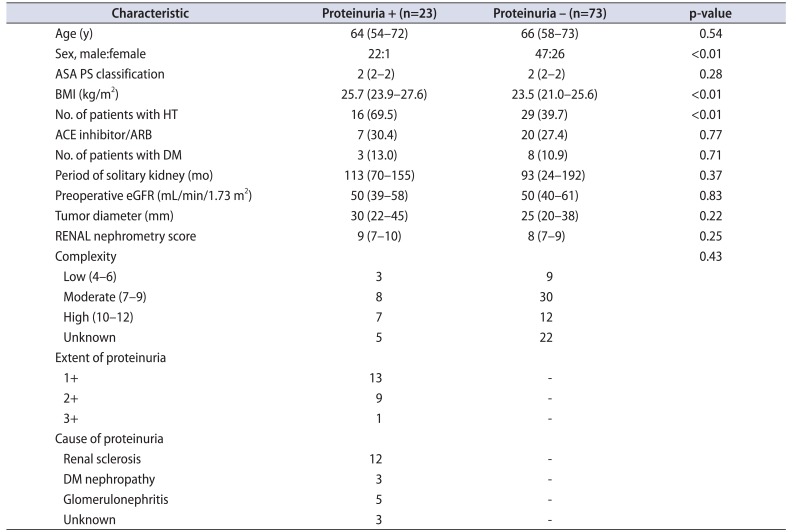
Values are presented as median (interquartile range) or number (%).
ASA PS, American Society of Anesthesiologists physical status; BMI, body mass index; HT, hypertension; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blockers; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; RENAL, radius, exophytic/endophytic, nearness of tumor to the collecting system or sinus, anterior/posterior, location relative to polar lines.
Table 2
Perioperative outcomes
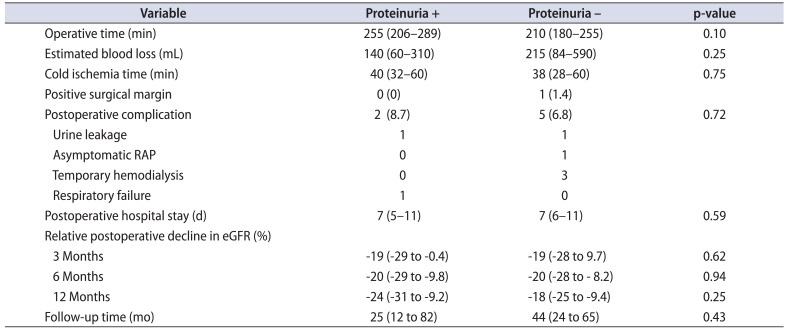
Table 3
Cox proportional hazards model regarding factors associated with postoperative decline in eGFR <30 mL/min/1.73 m2: univariate and multivariate analysis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download