Abstract
Male infertility affects men worldwide. Oxidative stress (OS), characterized by an overabundance of reactive oxygen species (ROS) or a deficiency of antioxidants, is one of the major causes of male infertility. OS causes damage at the molecular level, which impairs lipids, proteins, and DNA. The cyclic cascade of redox reactions weakens sperm function which leads to poor semen parameters and eventual sterility. There is a need for advanced diagnostic tests that can quickly and accurately detect OS. Most commonly used assays can only measure single constituents of OS. However, the MiOXSYS System introduces a new strategy to detect OS by measuring the oxidation-reduction potential (ORP)--a direct evaluation of the redox balance between ROS and antioxidants. The MiOXSYS System has shown promise as a diagnostic tool in the evaluation of male infertility. This review explores the concept of ORP, details the principle of the MiOXSYS System, and summarizes the findings in clinical studies that support ORP measurement in semen.
Approximately 7% of men worldwide are affected by male infertility, of which male factors contribute to 40%–50% of all infertility cases [1]. The basic semen analysis remains as the standard of care to initially evaluate male infertility despite its controversies in clinical effectiveness [23]. The test yields highly variable results from the same individual, inconsistent semen parameters among different observers, and no information about sperm dysfunctions at the molecular level. Additionally, the 2010 World Health Organization's reference values might not be applicable to all men as their inclusion criteria lacks diverse patient populations [4]. Therefore, more advanced tests of sperm function may assist in accurately diagnosing male infertility.
Oxidative stress (OS) is highly implicated in the pathogenesis of male infertility [5678]. OS occurs when production of reactive oxygen species (ROS) outweighs the concentration of antioxidants in human semen. Physiologically, ROS are vital for sperm maturation as they undergo capacitation, hyperactivation, acrosome reaction, and oocyte fusion [910]. However, excess ROS results in lipid peroxidation, DNA damage, and induction of apoptosis, all of which trigger a vicious cycle of OS [11]. Clinically, OS can translate into reduced fertilization rates, failure of implantation, impaired embryonic development, recurrent pregnancy loss and poor assisted reproductive technology (ART) outcomes [121314151617].
Early and accurate detection of OS ensures a better prognosis for infertile men. Currently available assays include chemiluminescence for ROS, total antioxidant capacity (TAC) for antioxidants, and malondialdehyde (MDA) assay for post hoc damage from lipid peroxidation. However, these tests carry certain disadvantages such as high cost, sophisticated instrumentation, large sample volumes, complex methodologies, and extensive technical training [1819202122] (Supplementary Table 1). Most importantly, they only capture a single dimension of OS, quantifying either ROS or antioxidants. Thus, a test that includes all of the constituents of OS may provide a better understanding of the true redox state and facilitate better management.
Measuring oxidation-reduction potential (ORP) is the latest advancement in male infertility diagnostics. ORP, also known as the redox balance, is a direct measure of OS as it describes the relative proportions of oxidants (ROS) to reductants (antioxidants). Previous clinical studies have been successful in evaluating ORP in the blood of patients with traumatic brain injury, stroke, metabolic syndrome, liver toxicity, sepsis, and exercise-induced OS [232425262728293031]. ORP measurement has recently been tested in human semen by a number of andrology laboratories to determine whether it is a reliable indicator of OS. The objective of this review is to (1) describe the causes and mechanisms of OS-induced damage in male infertility; (2) briefly discuss the mechanics of the MiOXSYS System, a device that measures ORP; (3) summarize the data produced by recent clinical studies; and (4) introduce studies in progress that may pave the way for utilization of ORP in clinical practice.
There are multiple endogenous and exogenous factors responsible for poor sperm quality in an infertile man. They cause infertility by producing a surplus of ROS targeted towards healthy spermatozoa. Examples of conditions that elevate ROS include: genital tract infections, varicocele, spinal cord injury, diabetes, obesity, tobacco smoking, alcohol use, recreational drug abuse, ionizing radiation, psychological stress, strenuous exercise, or air pollutants [323334] (Fig. 1). Two major sources of ROS are leukocytes via the hexose monophosphate shunt and immature spermatozoa at the level of the plasma membrane or mitochondria [35].
Spermatozoa are highly susceptible to ROS due to the specific composition of their plasma membrane. They contain polyunsaturated fatty acids, which are structurally unstable and highly prone to lipid peroxidation [36]. Electrophilic aldehyde byproducts, such as MDA, 4-hydroxynonenal, and acrolein, are generated from the peroxidative damage, which negatively affects DNA integrity, mitochondrial function, apoptosis, and cellular signaling [83738]. As aforementioned, the mitochondria are not only a target of ROS, but they are also a major source [39]. Thus, prolonged damage to the inner mitochondrial membrane will create a continuous cycle of ROS production that induces sterility. Overall, there is strong clinical evidence suggesting that OS is linked with male infertility as shown through various meta-analyses of infertile patients with OS risk factors (i.e., varicocele, chronic prostatitis, mobile phone use, cigarette smoking) [4041424344].
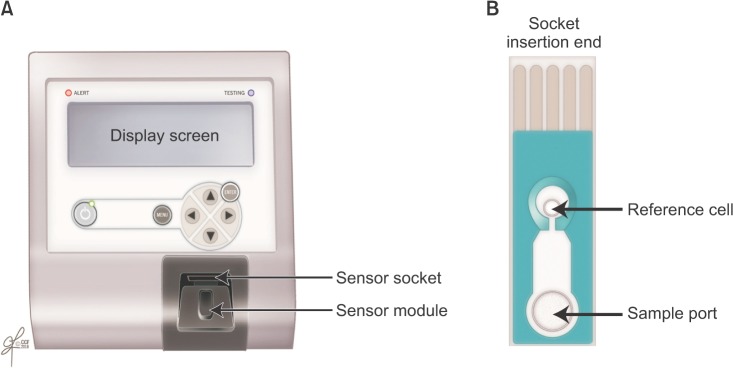
The MiOXSYS System is a novel technology designed to measure the transfer of electrons from reductants to oxidants in human semen samples. ORP is calculated with the Nernst equation: ORP=E°-RT/nF ln([Red]/[Ox]) where E°=standard reduction potential, R=universal gas constant, T=absolute temperature, n=number of moles of exchanged electrons, F=Faraday's constant, [Red]=concentration of reduced species, [Ox]=concentration of oxidized species [45]. To start the procedure, disposable test sensors—each equipped with three electrodes—are inserted into a galvanostatic analyzer (Fig. 2). Once the sample is applied onto the sample port, it will eventually reach the reference electrode, complete the electrochemical circuit, and signal the analyzer to emit a low voltage oxidizing current between electrodes. This current assists in generating an ORP from which the average is calculated from the final 10 seconds of the trial run [46]. The ORP value appears on the analyzer display screen as millivolts (mV), which parallels the degree of OS. A higher ORP corresponds to higher levels of oxidant activity [47].
Similar ORP devices exist in the market but are used to monitor water sanitation, metal finishing, and ozone treatment [4849505152]. To the best of our knowledge, the MiOXSYS System is the first ORP device to test human semen samples. It is specifically calibrated to detect human sperm at 0.1–400 mV. Requiring only 30 µL of sample volume and less than 5 minutes for the entire procedure, the MiOXSYS System yields an ORP value with a high sensitivity, specificity, and accuracy [535455565758]. Fresh or frozen semen and seminal plasma can be also be evaluated [59]. From a technical perspective, the MiOXSYS System is easy to use, inexpensive, less time consuming, and more efficient in providing reliable results. Factors that affect ORP measurement include advanced semen age, poor semen liquefaction, or repeated centrifugation [60616263].
Using the MiOXSYS System, the investigators at Cleveland Clinic's American Center for Reproductive Medicine began measuring ORP to detect OS in human semen samples. Recent clinical studies have generated significant data that enabled ORP measurement to quickly gain momentum as a robust diagnostic tool for male infertility. As a clarification, earlier studies looked at both static ORP (sORP) and antioxidant capacity reserve (cORP) [53]. sORP provides a composite measure of available oxidants and reductants whereas cORP denotes the amount of accessible reductants to combat OS. Due to more promising data, sORP became the topic of interest and was mostly referred to as “ORP” in later studies [54555658]. All studies were conducted with at least a power of 80% with statistical significance set at p<0.05. To calculate ORP (as normalized ORP), the raw ORP value (mV) was divided by the sperm concentration (sperm count × 106/mL). Normalized ORP was expressed as mV/106 sperm/mL. Additional information on the qualitative value of ORP measurement such as its unique ability in predicting abnormal semen parameters and identifying infertile men are elaborated with details of each clinical study published (Table 1).
Agarwal et al. [53] initiated a pilot study to measure ORP in human semen and seminal plasma. Another goal of the study was to observe the relationship between ORP and semen parameters across time. The investigators recruited a small cohort of 26 healthy controls and 33 infertile men. Semen samples were analyzed with a basic semen analysis per the 2010 World Health Organization guidelines and ORP measurement at 0 and 120 minutes after semen liquefaction. Compared to those of the controls, semen samples from infertile patients exhibited reduced motility and morphology (p<0.01). Additionally, they had elevated ORP levels in the seminal plasma at 120 minutes (p=0.036). Control and infertile patients were combined and categorized based on normal and abnormal motility (<40%). Individuals with poor sperm motility had lower concentrations, total count, and morphology (p<0.05). Their ORP levels were elevated in semen and seminal plasma at 120 minutes (p=0.035 and p=0.04, respectively). ORP was related to abnormal semen parameters as there was a significant inverse relationship of ORP with concentration and total sperm count at 0 and 120 minutes (p<0.05) (Supplementary Figs. 1,2,3,4). Via a receiver operating characteristic (ROC) curve analysis, an ORP cutoff value of 1.48 mV/106 sperm/mL in semen and 2.09 mV/106 sperm/mL in seminal plasma was proposed to aid in identifying abnormal semen samples, specifically those with <40% motility (Supplementary Fig. 5). Distributions of ORP values and the median in relation to the cutoffs are also displayed in Supplementary Fig. 5. The proposed ROC values were not statistically robust as it yielded low positive predictive values (PPVs) (45% in semen, 46.7% in seminal plasma). This most likely occurred due to a limited number of men with proven fertility in the control group. Including more individuals in the control group with similar baseline characteristics would increase the effectiveness of the assay. Overall, this study demonstrated that ORP can be detected in both semen and seminal plasma up to 120 minutes with the ability to perhaps distinguish abnormal semen quality.
In a second study, Agarwal et al. [54] further attempted to validate ORP as a surrogate marker in predicting abnormal semen. In this study, they enrolled a larger sample of 51 healthy controls (15 proven fertility and 36 unproven fertility) and 106 infertile patients (38 with varicocele, 13 idiopathic, 55 nonclassified). At baseline, the infertile patients had lower sperm concentrations, total sperm count, motility, and morphology (p≤0.001). ORP levels were significantly higher in infertile patients versus controls (6.22±1.10 mV/106 sperm/mL and 1.59±0.29 mV/106 sperm/mL, p=0.004). However, as a collective group, ORP was negatively correlated with concentration, total sperm count, motility, and morphology (p<0.01). ROC curve analysis yielded a normalized cut-off value of 1.36 mV/106 sperm/mL with a 69.6% sensitivity, 83.1% specificity, 85.3% PPV, 65.9% negative predictive value (NPV), and 75.2% accuracy (area under the curve [AUC]=0.770) (Fig. 3). Median ORP values of the infertile patients were above this cut-off value whereas those of the controls were below (p=0.004) (Fig. 4). The same trend was observed when comparing subcategories of patients and controls (Supplementary Fig. 6). Solely measuring ORP appeared to be effective in both determining excess OS in semen samples and differentiating between infertile patients and controls.
Intraobserver and interobserver reliability was tested by having different observers analyze multiple samples in replicates (Supplementary Fig. 7). The goal was to determine if ORP measurement provided more objective data as opposed to conventional semen analysis where high variability is an issue. This was expressed as a coefficient of variation (%CV). Variability for any one observer and across observers were 8.39% and 3.61%, respectively. Overall, this study supported ORP as a highly reproducible measurement and found that a normalized ORP cutoff of 1.36 mV/106 sperm/mL may have discriminative capabilities.
Although ORP values correlated strongly with semen parameters, this study did not further examine whether they were predictive of poor semen quality. This was an important question to answer since ORP in addition to abnormal semen parameters would theoretically be a more robust determination of OS status and male infertility.
Agarwal and Wang [55] sought to determine the predictive value of ORP and its relationship to semen parameters over time. Semen samples from 49 healthy controls and 194 infertile patients were obtained. Similar to previous studies, the infertile patients had abnormal semen parameters and elevated ORP levels compared with those of the controls (p<0.001). Significant negative correlations between ORP and certain semen parameters were also found in the infertile men (p<0.05). Using the 2010 WHO guidelines, semen samples from the infertile patients were arranged into normal and abnormal semen parameters (oligozoospermia, asthenozoospermia, and teratozoospermia). ORP levels were elevated in all abnormal semen parameters, the highest value belonging to the oligozoospermic group (p<0.001). In fact, ORP was the most predictive of oligozoospermic samples at a cutoff of 2.59 mV/106 sperm/mL with 88% sensitivity, 91.2% specificity, 90% PPV, 89.4% NPV, and 89.7% accuracy (AUC=0.754) (Fig. 5). This coincides with the negative correlation between ORP and concentration. This is also a logical observation since ORP is normalized to concentration.
Additional ROC curve analysis demonstrated that an ORP cutoff of 1.57 mV/106 sperm/mL achieving 70.4% sensitivity, 88.1% specificity, 95.5% PPV, 45.1% NPV, and 74.2% accuracy (AUC=0.809) can detect at least 1 abnormal semen parameter (p<0.001) (Fig. 6). There was a greater range of ORP values in infertile patients with abnormal sperm versus that of infertile patients with normal sperm. This suggests that ORP fluctuates as a reflection of abnormal semen parameters induced by OS. Nevertheless, the majority of patients with abnormal semen parameters had an ORP well above the cutoff value.
These reference values confirm prior data showing that ORP is related to semen parameters. Interestingly, because ORP best predicts oligozoospermia, it is very likely that OS strongly plays a pathologic role in this subset of patients [764]. It is important to not forget that OS contributes to other seminal abnormalities as well. This is reflected through different ORP cutoffs for each abnormal semen parameter. This study reveals that a combined approach of considering ORP and at least 1 abnormal semen parameter (especially concentration) is more robust in identifying OS than ORP alone.
To test the relationship between ORP and semen parameters over time, 28 infertile patients underwent repeat semen analyses and ORP measurements at baseline and after 3–5 months. At follow-up, sperm concentration, total motility, and ORP had significantly improved (Supplementary Table 2). This shows that sperm concentration and total motility increase as ORP decreases. Lastly, whether ORP can be used to monitor male infertility due to an infectious agent or inflammatory condition remains unclear. Leukocytes were present in 9 infertile semen samples as verified by the Endtz test and were treated with 200-mg doxycycline daily for 2–3 weeks. Although leukocytes were essentially eliminated, ORP did not show a statistically significant decline.
Agarwal et al. [56] then evaluated the ability of additional combinations of semen parameters and ORP to identify OS regardless of fertility status. The authors also compared ORP levels among different categories of abnormal semen parameters arranged by the 2010 WHO criteria. This study consisted of 15 healthy controls with proven fertility and 293 infertile patients. Comparing infertile patients with controls, there were significant differences in all semen parameters (p<0.05), except for ORP. However, as expected, ORP was negatively correlated with sperm concentration and motility (p<0.0001). Afterwards, healthy and infertile semen samples were grouped altogether and categorized based on abnormal semen parameters (oligozoospermic [OZ], asthenozoospermic [AZ], teratozoospermic [TZ], and oligoasthenoteratozoospermic [OAT]). Normal semen samples were labeled normozoospermic (NZ).
ORP levels were significantly higher in the groups with abnormal semen parameters than in the NZ samples (p<0.0001). Also, ORP was highly predictive of AZ due to the high specificity (86.3%) and PPV (75.3%). This was not the case in regards to TZ. Interestingly, although ORP had a high specificity in predicting OAT, it had a low PPV. This means that an ORP value above the proposed OAT cutoff did not definitively indicate the presence of OAT due to the presence of many false positives. Similar to prior research findings, ORP was best at predicting OZ with a cutoff of 2.63 mV/106 sperm/mL 81.5% sensitivity, 92.7% specificity, 89.1% PPV, 87.2% NPV and AUC=0.919.
Lastly, an ORP cutoff of 2.7 mV/106 sperm/mL can detect at least 2 abnormal semen parameters achieving 64.6% sensitivity, 83.9% specificity, 75.7% PPV, 75.4% NPV and AUC=0.809 (p<0.0001). With this data, clinicians may use a combination of ORP and 2 other abnormal semen parameters to help identify OS rather than using ORP alone. The study results confirmed that ORP measurement is a reproducible test that is highly predictive of abnormal semen parameters regardless of fertility status. Identifying ORP provides a quick composite picture of OS status and abnormal semen parameters in just 1 patient visit. Therefore, the need for multiple follow-ups with patients are not necessary.
To further test ORP's consistency, a number of studies have used the MiOXSYS System in different ethnic populations at different geographical locations. Agarwal et al. [57] conducted a multicenter retrospective study that compared data from 2 andrology laboratories located in the USA and Qatar for 12 months. Cleveland Clinic, Cleveland, USA, recruited 51 healthy fertile controls and 194 infertile patients. A large tertiary hospital located in Doha, Qatar, recruited 50 fertile controls and 400 infertile patients. The sample population was analyzed as a combined dataset and as individual datasets according to institution. In both data sets and the combined dataset, sperm concentration, total motility, progressive motility, and normal morphological forms were significantly lower and ORP significantly higher in the infertile patients than in the controls (p<0.05). Between the infertile patients of the Cleveland Clinic and Doha, there were no significant differences in ORP or semen parameters except for progressive motility and morphology. Between control patients of the Cleveland Clinic and Doha, there was only a significant difference in morphology (p=0.004).
Data from the combined population and combined number of infertile patients of both institutions were divided into normal and abnormal semen groups according to the 2010 WHO semen parameters. ORP was elevated in both abnormal semen groups (p<0.001) (Tables 2, 3). When combining both study populations, ROC curve analysis generated an ORP cutoff value of 1.42 mV/106 sperm/mL that was able to differentiate fertile from infertile semen groups with 60.6% sensitivity, 74.3% specificity, 93.3% PPV, 24.3% NPV, and 62.6% accuracy (Supplementary Fig. 8). This ORP value persisted even with variations of baseline characteristics such as smoking status, leukocytospermia, age, body mass index, and number of abstinence days.
Arafa et al. [58] provided supporting data from Qatar through a prospective study of 365 infertile patients and 50 fertile controls. Decreased semen parameters in all categories and elevated ORP levels were found in the infertile patients (p<0.001) when compared with fertile controls. ORP also showed no significant correlation with age, BMI, or days of sexual abstinence despite differences in those baseline demographics. ROC curve analysis provided an ORP cutoff of 1.38 mV/106 sperm/mL to differentiate normal from abnormal semen quality with a 63.3% sensitivity, 87.8% specificity, 97.6% PPV, 23.2% NPV, and 66% accuracy. A cutoff of 1.41 mV/106 sperm/mL was able to distinguish fertile from infertile men with an accuracy that was better than that of other WHO semen parameters except for progressive motility—a parameter that is quite subjective due to its high intra- and interindividual variability (Table 4).
By enrolling a large number of patients, these studies were instrumental in providing reliable ORP cutoffs that can identify abnormal semen samples and infertile men. Since these values are similar to the ones proposed in previous studies, measuring ORP is a reliable and reproducible technique when analyzing semen samples in diverse populations.
ORP's role as a surrogate marker to aid in the diagnosis of male infertility is under investigation by numerous groups. The following topics were discussed primarily through some recent abstracts presented at national and international medical conferences within the past 2 years. Many of the studies attempted to improve upon previously published papers by recruiting larger sample sizes treating leukocytospermia, monitoring ORP response to treatment, classifying patients according to etiologies of male infertility, and evaluating female factor infertility in the patients' female partners.
There is convincing evidence that further supports the relationship between ORP and semen parameters. According to Roychoudhury et al. [65], an ORP cutoff of 1.23 mV/106 sperm/mL could identify over 90% of healthy semen samples which was defined as sperm that meets all of the 2010 WHO normal semen parameter criteria. In fact, Elbardisi et al. [66] found that semen with 1 or more poor parameters had higher ORP values than semen that met all of WHO criteria (p<0.05). ORP increased with abnormal semen parameters. Lastly, an ORP cutoff of 1.635 mV/106 sperm/mL had a 98.6% chance of predicting semen with at least 1 or more abnormal semen parameters. Toor et al. [67] observed that ORP was elevated in semen that had a low sperm concentration, low total sperm count, and low sperm motility (p<0.05). There was also a negative correlation with ORP and these parameters (p<0.05). Lastly, Agarwal et al. [68] conducted a multicenter study at 9 institutions around the world with 2010 recruited participants. An ORP cutoff value of 1.34 mV/106 sperm/mL was able to identify samples with abnormal semen parameters with 58% sensitivity, 85% specificity, 96% PPV, 42% NPV, and AUC=0.757. The cutoffs and relationships observed in these studies were similar to the ones established by Cleveland Clinic researchers.
ORP's relationship to sperm morphology is inconsistent. This adds to the controversy of whether to use sperm morphology as an indicator of sperm function [697071]. The aforementioned clinical studies found weak evidence in supporting ORP to predict teratozoospermia [5556]. However, Arafa et al. [72] suggested that a cutoff of 3.29 mV/106 sperm/mL was able to reliably predict abnormal morphology with a 55.6% sensitivity, 89.1% specificity, 85.7.1% PPV, 63.1% NPV and 71% accuracy (AUC=0.90). Additionally, Ayaz et al. [73] found that although sperm morphological abnormalities were present in both fertile and infertile men, they were often found in combination with other semen parameter abnormalities in infertile semen samples. ORP also increased in infertile semen samples as the percentage of sperm neck abnormalities increased.
Total motile sperm count (TMSC) is an excellent semen parameter in predicting severity of male infertility [7475]. Al Said et al. [76] sought to determine a correlation between ORP and TMSC, in hopes that these 2 parameters could provide additional information in the evaluation of male infertility. A significant negative correlation was found (p<0.001). Also, ROC curve analysis showed that an abnormal TMSC (>20 million) can be best predicted with an ORP cutoff of 2.34 mV/106 sperm/mL with a 83.5% sensitivity, 82.5% specificity, 82.9% PPV, 81.4% NPV, and 79.9% accuracy (AUC=0.9). Because of the promising data, this study advocates for concurrent measurement of TMSC and ORP to evaluate male infertility.
Sperm DNA fragmentation (SDF) is most commonly seen in infertile men, which if not detected early, can impair fertilization, embryo development, and successful clinical pregnancy [77787980]. Arafa et al. [81] conducted a prospective study of 312 patients and reported that DNA fragmentation was negatively correlated with total/progressive motility (p<0.001) and positively correlated with abnormal morphology, ORP, and age (p<0.001). Elevated ORP levels were also seen in the semen of the high SDF group compared to that of the normal SDF group (4.03±0.61 mV/106 sperm/mL and 2.14±0.14 mV/106 sperm/mL, respectively, p<0.001).
In a cross sectional study of 1,162 patients, Majzoub et al. [82] observed a positive correlation between the percentage of abnormal sperm heads and levels of ORP (p<0.001) and SDF (p<0.001). This confirms that OS is highly implicated in sperm DNA damage, especially SDF [838485]. In regards to reproductive outcomes, Ayaz et al. [86] observed that the clinical pregnancy rate was higher in patients in a low ORP group (<1.36 mV/106 sperm/mL) than in those in a high ORP group (>1.36 mV/106 sperm/mL) (p=0.006). For ART purposes, monitoring ORP allows for better sperm selection and preparation [87]. Overall, ORP not only detects OS-associated damage, but also provides clues on prognosis which fully informs the clinician on an individual's clinical status.
Varicocele is the most common correctable cause of male infertility. Although there are many possible mechanisms of varicocele-induced injury, OS appears to be the main culprit [88]. Agarwal and colleagues observed that ORP levels were higher and semen parameters (concentration, total motility, and morphology) were lower in varicocele patients than in healthy controls (p=0.001) [8990]. ORP was also negatively correlated with concentration, total motility and morphology (p=0.001) [90]. Currently, there is no consensus on the differences of ORP among grades of varicocele—one study found no difference whereas another study stated that patients with a grade 3 varicocele had the highest levels [9091]. Lastly, when compared to patients of idiopathic infertility, varicocele patients had significantly lower sperm concentration, fewer normal morphologic forms, and higher seminal ORP (p<0.05) [92].
Genitourinary infections are a major contributor to OS-induced male infertility. Accumulated leukocytes in the seminal plasma (leukocytospermia) increase ROS production and lead to abnormal semen parameters [9394]. In a preliminary study, Sikka and colleagues [9596] proposed monitoring ORP as an indicator of OS in leukocytospermia because ORP values paralleled the levels of certain biomarkers of active inflammation such as the Toll-like receptor 4 and cyclooxygenase 2.
A clinician should measure ORP in all patients who present to the infertility clinic as an adjunct to the basic semen analysis. As demonstrated in clinical studies, the excellent results show that ORP can provide valuable information about sperm function and a man's fertilization capabilities. This highlights ORP measurement as an important ancillary tool for male infertility evaluation. ORP can be measured to assess patients' initial OS status and to help guide therapeutic interventions. Theoretically, ORP should decrease when the underlying cause of OS is eradicated. Varicocelectomy, antioxidant supplementation, antibiotic treatment, and lifestyle modifications are examples of treatments that may be beneficial in alleviating OS and improving reproductive outcomes [979899100]. This suggests that ORP measurement is perhaps most beneficial for patients with varicocele, idiopathic infertility, and OS-inducing lifestyle habits. Nonetheless, further studies are needed to determine if ORP can be used to longitudinally monitor treatment progress in patients with specific clinical conditions.
Male infertility is a multifactorial condition in which OS plays a central role. Newer measures such as ORP represent an objective and accurate method that can reliably identify abnormal semen quality and differentiate fertile from infertile men. The ORP test is also a cost-effective and convenient option for patients undergoing evaluation for male factor infertility. ORP measurement has not yet been incorporated into standard clinical practice due to an incomplete understanding of the clinical indications. Its ability to discriminate across different clinical conditions and to monitor therapeutic effectiveness remains unclear. Also, the current evidence is not yet strong enough to advocate for widespread use of ORP as a stand-alone test. Thus, ORP measurement by the MiOXSYS System provides a novel diagnostic method that may be used in conjunction to routine semen analysis with hopes of positively affecting the lives of men burdened by infertility.
ACKNOWLEDGMENTS
The authors want to thank the following individuals for their critical review and suggestions on our manuscript: Chak Lam Cho, MD; Ahmad Majzoub, MD; Ralf Henkel, PhD; Rakesh Sharma, PhD. Editorial support was provided by Ms. Amy Moore. The study was supported by funds from the American Center for Reproductive Medicine, Cleveland Clinic Foundation, Cleveland, OH, USA.
References
1. Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: a review of literature. J Hum Reprod Sci. 2015; 8:191–196. PMID: 26752853.

2. Esteves SC. Clinical relevance of routine semen analysis and controversies surrounding the 2010 World Health Organization criteria for semen examination. Int Braz J Urol. 2014; 40:443–453. PMID: 25254609.

3. Esteves SC, Hamada A, Kondray V, Pitchika A, Agarwal A. What every gynecologist should know about male infertility: an update. Arch Gynecol Obstet. 2012; 286:217–229. PMID: 22392488.

4. Esteves SC, Zini A, Aziz N, Alvarez JG, Sabanegh ES Jr, Agarwal A. Critical appraisal of World Health Organization's new reference values for human semen characteristics and effect on diagnosis and treatment of subfertile men. Urology. 2012; 79:16–22. PMID: 22070891.

5. Aitken RJ, Clarkson JS. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil. 1987; 81:459–469. PMID: 2828610.

6. Alvarez JG, Touchstone JC, Blasco L, Storey BT. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity. J Androl. 1987; 8:338–348. PMID: 2822642.

7. Aitken RJ, Clarkson JS, Hargreave TB, Irvine DS, Wu FC. Analysis of the relationship between defective sperm function and the generation of reactive oxygen species in cases of oligozoospermia. J Androl. 1989; 10:214–220. PMID: 2501260.

8. Bisht S, Dada R. Oxidative stress: major executioner in disease pathology, role in sperm DNA damage and preventive strategies. Front Biosci (Schol Ed). 2017; 9:420–447. PMID: 28410127.
9. de Lamirande E, Jiang H, Zini A, Kodama H, Gagnon C. Reactive oxygen species and sperm physiology. Rev Reprod. 1997; 2:48–54. PMID: 9414465.

10. Du Plessis SS, Agarwal A, Halabi J, Tvrda E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J Assist Reprod Genet. 2015; 32:509–520. PMID: 25646893.

11. Aitken RJ. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol Reprod Dev. 2017; 84:1039–1052. PMID: 28749007.

12. Imam SN, Shamsi MB, Kumar K, Deka D, Dada R. Idiopathic recurrent pregnancy loss: role of paternal factors; a pilot study. J Reprod Infertil. 2011; 12:267–276. PMID: 23926513.
13. Zidi-Jrah I, Hajlaoui A, Mougou-Zerelli S, Kammoun M, Meniaoui I, Sallem A, et al. Relationship between sperm aneuploidy, sperm DNA integrity, chromatin packaging, traditional semen parameters, and recurrent pregnancy loss. Fertil Steril. 2016; 105:58–64. PMID: 26493117.

14. Henkel R, Kierspel E, Hajimohammad M, Stalf T, Hoogendijk C, Mehnert C, et al. DNA fragmentation of spermatozoa and assisted reproduction technology. Reprod Biomed Online. 2003; 7:477–484. PMID: 14656411.

15. Du Plessis SS, Makker K, Desai NR, Agarwal A. Impact of oxidative stress on IVF. Expert Rev Obstet Gynecol. 2008; 3:539–554.

16. Yang Q, Zhao F, Hu L, Bai R, Zhang N, Yao G, et al. Effect of paternal overweight or obesity on IVF treatment outcomes and the possible mechanisms involved. Sci Rep. 2016; 6:29787. PMID: 27412918.

17. Opuwari CS, Henkel RR. An update on oxidative damage to spermatozoa and oocytes. Biomed Res Int. 2016; 2016:9540142. PMID: 26942204.

18. Agarwal A, Allamaneni SS, Said TM. Chemiluminescence technique for measuring reactive oxygen species. Reprod Biomed Online. 2004; 9:466–468. PMID: 15511350.

19. Said TM, Kattal N, Sharma RK, Sikka SC, Thomas AJ Jr, Mascha E, et al. Enhanced chemiluminescence assay vs colorimetric assay for measurement of the total antioxidant capacity of human seminal plasma. J Androl. 2003; 24:676–680. PMID: 12954657.

20. Sharma RK, Pasqualotto FF, Nelson DR, Thomas AJ Jr, Agarwal A. The reactive oxygen species-total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum Reprod. 1999; 14:2801–2807. PMID: 10548626.

21. Grotto D, Santa Maria LD, Boeira S, Valentini J, Charão MF, Moro AM, et al. Rapid quantification of malondialdehyde in plasma by high performance liquid chromatography-visible detection. J Pharm Biomed Anal. 2007; 43:619–624. PMID: 16949242.

22. Agarwal A, Roychoudhury S, Bjugstad KB, Cho CL. Oxidation-reduction potential of semen: what is its role in the treatment of male infertility? Ther Adv Urol. 2016; 8:302–318. PMID: 27695529.

23. Shapiro HM. Redox balance in the body: an approach to quantitation. J Surg Res. 1972; 13:138–152. PMID: 4343247.

24. Bjugstad KB, Rael LT, Levy S, Carrick M, Mains CW, Slone DS, et al. Oxidation-reduction potential as a biomarker for severity and acute outcome in traumatic brain injury. Oxid Med Cell Longev. 2016; 2016:6974257. PMID: 27642494.

25. Bjugstad KB, Fanale C, Wagner J, Jensen J, Salottolo K, Rael LT, Bar-Or D. A 24 h delay in the redox response distinguishes the most severe stroke patients from less severe stroke patients. J Neurol Neurophys. 2016; 7:1000395.

26. Rael LT, Bar-Or R, Aumann RM, Slone DS, Mains CW, Bar-Or D. Oxidation-reduction potential and paraoxonase-arylesterase activity in trauma patients. Biochem Biophys Res Commun. 2007; 361:561–565. PMID: 17662690.

27. Rael LT, Bar-Or R, Mains CW, Slone DS, Levy AS, Bar-Or D. Plasma oxidation-reduction potential and protein oxidation in traumatic brain injury. J Neurotrauma. 2009; 26:1203–1211. PMID: 19317602.

28. Bobe G, Cobb TJ, Leonard SW, Aponso S, Bahro CB, Koley D, et al. Increased static and decreased capacity oxidation-reduction potentials in plasma are predictive of metabolic syndrome. Redox Biol. 2017; 12:121–128. PMID: 28222379.

29. Bar-Or R, Rael LT, Curtis CG, Mains CW, Slone DS, Bar-Or D. Raman spectral signatures of human liver perfusates correlate with oxidation reduction potential. Mol Med Rep. 2009; 2:175–180. PMID: 21475809.

30. Spanidis Y, Goutzourelas N, Stagos D, Kolyva AS, Gogos CA, Bar-Or D, et al. Assessment of oxidative stress in septic and obese patients using markers of oxidation-reduction potential. In Vivo. 2015; 29:595–600. PMID: 26359419.
31. Stagos D, Goutzourelas N, Bar-Or D, Ntontou AM, Bella E, Becker AT, et al. Application of a new oxidation-reduction potential assessment method in strenuous exercise-induced oxidative stress. Redox Rep. 2015; 20:154–162. PMID: 25494543.

32. Aitken RJ, Gibb Z, Baker MA, Drevet J, Gharagozloo P. Causes and consequences of oxidative stress in spermatozoa. Reprod Fertil Dev. 2016; 28:1–10. PMID: 27062870.

33. Sabeti P, Pourmasumi S, Rahiminia T, Akyash F, Talebi AR. Etiologies of sperm oxidative stress. Int J Reprod Biomed (Yazd). 2016; 14:231–240. PMID: 27351024.

34. de Lamirande E, Leduc BE, Iwasaki A, Hassouna M, Gagnon C. Increased reactive oxygen species formation in semen of patients with spinal cord injury. Fertil Steril. 1995; 63:637–642. PMID: 7851599.
35. Aprioku JS. Pharmacology of free radicals and the impact of reactive oxygen species on the testis. J Reprod Infertil. 2013; 14:158–172. PMID: 24551570.
36. Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003; 79:829–843. PMID: 12749418.

37. Łuczaj W, Skrzydlewska E. DNA damage caused by lipid peroxidation products. Cell Mol Biol Lett. 2003; 8:391–413. PMID: 12813574.
38. Aitken RJ, Whiting S, De Iuliis GN, McClymont S, Mitchell LA, Baker MA. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J Biol Chem. 2012; 287:33048–33060. PMID: 22851170.

39. Cui H, Kong Y, Zhang H. Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct. 2012; 2012:646354. PMID: 21977319.

40. Agarwal A, Allamaneni SS, Nallella KP, George AT, Mascha E. Correlation of reactive oxygen species levels with the fertilization rate after in vitro fertilization: a qualified meta-analysis. Fertil Steril. 2005; 84:228–231. PMID: 16009190.

41. Agarwal A, Prabakaran S, Allamaneni SS. Relationship between oxidative stress, varicocele and infertility: a meta-analysis. Reprod Biomed Online. 2006; 12:630–633. PMID: 16790111.

42. Condorelli RA, Russo GI, Calogero AE, Morgia G, La Vignera S. Chronic prostatitis and its detrimental impact on sperm parameters: a systematic review and meta-analysis. J Endocrinol Invest. 2017; 5. 09. [Epub]. DOI: 10.1007/s40618-017-0684-0.

43. Adams JA, Galloway TS, Mondal D, Esteves SC, Mathews F. Effect of mobile telephones on sperm quality: a systematic review and meta-analysis. Environ Int. 2014; 70:106–112. PMID: 24927498.

44. Sharma R, Harlev A, Agarwal A, Esteves SC. Cigarette smoking and semen quality: a new meta-analysis examining the effect of the 2010 World Health Organization laboratory methods for the examination of human semen. Eur Urol. 2016; 70:635–645. PMID: 27113031.

45. R Bar-OrD Bar-OrLT Rael. Aytu Bioscience, Inc.Method and apparatus for measuring oxidation-reduction potential. United States patent. US 9,528,959. 2016. 12. 27.
46. Rael LT, Bar-Or R, Kelly MT, Carrick MM, Bar-Or D. Assessment of oxidative stress in patients with an isolated traumatic brain injury using disposable electrochemical test strips. Electroanalysis. 2015; 27:2567–2573.

47. Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002; 30:620–650. PMID: 12512863.
48. Victorin K, Hellström KG, Rylander R. Redox potential measurements for determining the disinfecting power of chlorinated water. J Hyg (Lond). 1972; 70:313–323. PMID: 4555890.

49. Bergendahl JA, Stevens L. Oxidation reduction potential as a measure of disinfection effectiveness for chlorination of wastewater. Environ Prog Sustain Energy. 2005; 24:214–222.

50. Ye Z, Wang S, Chen T, Gao W, Zhu S, He J, et al. Inactivation mechanism of escherichia coli induced by slightly acidic electrolyzed water. Sci Rep. 2017; 7:6279. PMID: 28740247.

51. McFarland MJ, Glarborg C, Ross MA. Chemical treatment of chelated metal finishing wastes. Water Environ Res. 2012; 84:2086–2089. PMID: 23342939.

52. Hjorth M, Pedersen CØ, Feilberg A. Redox potential as a means to control the treatment of slurry to lower HS emissions. Sensors (Basel). 2012; 12:5349–5362. PMID: 22778588.
53. Agarwal A, Sharma R, Roychoudhury S, Du Plessis S, Sabanegh E. MiOXSYS: a novel method of measuring oxidation reduction potential in semen and seminal plasma. Fertil Steril. 2016; 106:566–573.e10. PMID: 27260688.

54. Agarwal A, Roychoudhury S, Sharma R, Gupta S, Majzoub A, Sabanegh E. Diagnostic application of oxidation-reduction potential assay for measurement of oxidative stress: clinical utility in male factor infertility. Reprod Biomed Online. 2017; 34:48–57. PMID: 27839743.

55. Agarwal A, Wang SM. Clinical relevance of oxidation-reduction potential in the evaluation of male infertility. Urology. 2017; 104:84–89. PMID: 28214572.

56. Agarwal A, Henkel R, Sharma R, Tadros N, Sabanegh E. Determination of seminal oxidation-reduction potential (ORP) as an easy and cost-effective clinical marker of male infertility. Andrologia. 2017; Forthcoming.

57. Agarwal A, Arafa M, Chandrakumar R, Majzoub A, AlSaid S, Elbardisi H. A multicenter study to evaluate oxidative stress by oxidation-reduction potential, a reliable and reproducible method. Andrology. 2017; 5:939–945. PMID: 28726302.

58. Arafa M, Agarwal A, AlSaid S, Majzoub A, Sharma R, Bjugstad KB, et al. Semen quality and infertility status can be identified through measures of oxidation-reduction potential. Andrologia. 2017; 8. 03. [Epub]. DOI: 10.1111/and.12881.

59. Agarwal A, Sharma R, Henkel R, Roychoudhury S, Sikka SC, duPlessis S, et al. Cumene hydroperoxide induced changes in oxidation-reduction potential in fresh and frozen seminal ejaculates. Andrologia. 2017; 3. 15. [Epub]. DOI: 10.1111/and.12796.

60. Shekarriz M, Thomas AJ Jr, Agarwal A. Effects of time and sperm concentration on reactive oxygen species formation in human semen. Arch Androl. 1995; 34:69–75. PMID: 7786090.

61. Du Plessis SS, Gokul S, Agarwal A. Semen hyperviscosity: causes, consequences, and cures. Front Biosci (Elite Ed). 2013; 5:224–231. PMID: 23276984.
62. Aitken RJ, Clarkson JS. Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques. J Androl. 1988; 9:367–376. PMID: 3215823.

63. Li Z, Zhou Y, Liu R, Lin H, Liu W, Xiao W, et al. Effects of semen processing on the generation of reactive oxygen species and mitochondrial membrane potential of human spermatozoa. Andrologia. 2012; 44:157–163. PMID: 21729130.

64. Agarwal A, Mulgund A, Sharma R, Sabanegh E. Mechanisms of oligozoospermia: an oxidative stress perspective. Syst Biol Reprod Med. 2014; 60:206–216. PMID: 24815996.

65. Roychoudhury C, Dorsey C, Choudhury BP, Kushal KK. Oxidation-reduction potential can help distinguish semen samples under oxidative stress. Poster presented at: 33rd Annual European Society for Health and Reproduction Conference. 2017 Jul 3-5; Geneva, Switzerland.
66. Elbardisi H, Arafa M, Agarwal A, AlSaid S, Majzoub A, Keyvanifard F, et al. Seminal fluid static oxidation reduction potential is lower in seminal fluid that meets WHO criteria for fertile men. Poster presented at: 32nd Annual European Society for Health and Reproduction Conference. 2016 Jul 3-6; Helsinki, Finland.
67. Toor JS, Daniels L, Yafi FA, Hellstrom W, Sikka SC. Semen oxidative reduction potential (ORP) is related to abnormal semen parameters in male factor infertility. Poster presented at: 41st Annual America Society of Andrology Conference. 2016 Apr 2-5; New Orleans, LA, USA.
68. Agarwal A, Elbardisi H, Arafa M, Okada H, Suzuki K, Homa S, et al. Multi-center evaluation of oxidation reduction potential assay in the infertile male. Poster presented at: 73rd Annual American Society for Reproductive Medicine Conference. 2017 Oct 28-Nov 1; San Antonio, TX, USA.
69. Kovac JR, Smith RP, Cajipe M, Lamb DJ, Lipshultz LI. Men with a complete absence of normal sperm morphology exhibit high rates of success without assisted reproduction. Asian J Androl. 2017; 19:39–42. PMID: 27751992.

70. Shabtaie SA, Gerkowicz SA, Kohn TP, Ramasamy R. Role of abnormal sperm morphology in predicting pregnancy outcomes. Curr Urol Rep. 2016; 17:67. PMID: 27469478.

71. Franken DR. How accurate is sperm morphology as an indicator of sperm function? Andrologia. 2015; 47:720–723. PMID: 25130990.

72. Arafa M, Elbardisi H, Agarwal A, AlSaid S, Majzoub A, Al RumaihiK, et al. Oxidation-reduction potential: a new predictor for sperm morphology in infertile men. Poster presented at: 32nd Annual European Society for Health and Reproduction Conference. 2016 Jul 3-6; Helsinki, Finland.
73. Ayaz A, Bjugstad KB, Bar-Or D, Armagan A. Infertile men have a redox balance that distinguishes them from fertile men. Poster presented at: 72nd Annual American Society for Reproductive Medicine Conference. 2016 Oct 15-19; Salt Lake City, UT, USA.
74. Hamilton JA, Cissen M, Brandes M, Smeenk JM, de Bruin JP, Kremer JA, et al. Total motile sperm count: a better indicator for the severity of male factor infertility than the WHO sperm classification system. Hum Reprod. 2015; 30:1110–1121. PMID: 25788568.

75. Borges E Jr, Setti AS, Braga DP, Figueira RC, Iaconelli A Jr. Total motile sperm count has a superior predictive value over the WHO 2010 cut-off values for the outcomes of intracytoplasmic sperm injection cycles. Andrology. 2016; 4:880–886. PMID: 27152971.

76. AlSaid S, Majzoub A, Arafa M, ElBardisi H, Agarwal A, AlRumaihi K. Oxidation-reduction potential: a valuable tool for male fertility evaluation. Poster presented at: 33rd Annual European Society for Health and Reproduction Conference. 2017 Jul 3-5; Geneva, Switzerland.
77. Saleh RA, Agarwal A, Nada EA, El-Tonsy MH, Sharma RK, Meyer A, et al. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril. 2003; 79(Suppl 3):1597–1605. PMID: 12801566.

78. Simon L, Proutski I, Stevenson M, Jennings D, McManus J, Lutton D, et al. Sperm DNA damage has a negative association with live-birth rates after IVF. Reprod Biomed Online. 2013; 26:68–78. PMID: 23200202.

79. Lewis SE, John Aitken R, Conner SJ, Iuliis GD, Evenson DP, Henkel R, et al. The impact of sperm DNA damage in assisted conception and beyond: recent advances in diagnosis and treatment. Reprod Biomed Online. 2013; 27:325–337. PMID: 23948450.

80. Agarwal A, Majzoub A, Esteves SC, Ko E, Ramasamy R, Zini A. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol. 2016; 5:935–950. PMID: 28078226.

81. Arafa M, ElBardisi H, Majzoub A, Al Said S, AlNawasra H, Khalafalla K, et al. Correlation of sperm DNA fragmentation and seminal oxidation-reduction potential in infertile men. Poster presented at: 33rd Annual European Society for Health and Reproduction Conference. 2017 Jul 3-5; Geneva, Switzerland.
82. Majzoub A, Arafa M, Elbardisi H, Al Said S, Agarwal A, Al Rumaihi K. Oxidation-reduction potential and sperm DNA fragmentation levels in sperm morphologic anomalies. Poster presented at: 33rd Annual European Society for Health and Reproduction Conference. 2017 Jul 3-5; Geneva, Switzerland.
83. Dorostghoal M, Kazeminejad SR, Shahbazian N, Pourmehdi M, Jabbari A. Oxidative stress status and sperm DNA fragmentation in fertile and infertile men. Andrologia. 2017; 1. 26. [Epub]. DOI: 10.1111/and.12762.

84. Ionmmiello VM, Albani E, Di Rosa A, Marras A, Menduni F, Morreale G, et al. Ejaculate oxidative stress is related with sperm DNA fragmentation and round cells. Int J Endocrinol. 2015; 2015:321901. PMID: 25802519.
85. Bisht S, Faiq M, Tolahunase M, Dada R. Oxidative stress and male infertility. Nat Rev Urol. 2017; 14:470–485. PMID: 28508879.

86. Ayaz A, Balaban B, Sikka S, Isiklar A, Tasdemir M, Urman B. Effect of seminal ORP value on embryo quality and clinical pregnancy rate. Poster presented at: 33rd Annual European Society for Health and Reproduction Conference. 2017 Jul 3-5; Geneva, Switzerland.
87. Agarwal A, Roychoudhury S, Esteves S, Rosas IM, Sharma R, Gupta S. Oxidation-reduction potential (ORP) of spermatazoa selected for intracytoplasmic sperm injection (ICSI) after exposure to polyvinylpyrrolidone (PVP) and hyaluronic acid (HA). Poster presented at: 32nd Annual European Society for Health and Reproduction Conference. 2016 Jul 3-6; Helsinki, Finland.
88. Jensen CF, Ostergren P, Dupree JM, Ohl D, Sonksen J, Fode M. Varicocele and male infertility. Nat Rev Urol. 2017; 14:523–533. PMID: 28675168.

89. Agarwal A, Wang SM, Tadros N, Sabanegh E. Involvement of oxidation reduction potential in the pathophysiology of male infertility patients with varicocele. Poster presented at 112th Annual American Urological Association Conference. 2017 May 12-16; Boston, MA, USA.
90. Arafa M, ElBardisi H, Majzoub A, AlSaid S, Jaber A, Khalafalla K, et al. Role of oxidation reduction potential in varicocele associated male infertility. Poster presented at 112th American Urological Association Conference. 2017 May 12-16; Boston, MA, USA.
91. Agarwal A, Majzoub A, Roychoudhury S, Arafa MM. Oxidation reduction potential: a novel marker of varicocele pathophysiology. Poster presented at: 72nd Annual American Society for Reproductive Medicine Conference. 2016 Octr 15-19; Salt Lake City, UT, USA.
92. Saleh R, Agarwal A. High levels of seminal oxidation-reduction potential (ORP) in infertile men with clinical varicocele. Poster presented at: 33rd Annual European Society for Health and Reproduction Conference. 2017 Jul 3-5; Geneva, Switzerland.
93. Saleh RA, Agarwal A, Kandirali E, Sharma RK, Thomas AJ, Nada EA, et al. Leukocytospermia is associated with increased reactive oxygen species production by human spermatazoa. Fertil Steril. 2002; 78:1215–1224. PMID: 12477515.
94. Alvarez JG, Sharma RK, Ollero M, Saleh RA, Lopez MC, Thomas AJ Jr, et al. Increased DNA damage in sperm from leukocytospermic semen samples as determined by sperm chromatin structure assay. Fertil Steril. 2002; 78:319–329. PMID: 12137869.
95. Hagan S, Khurana N, Chandra S, Abdel-Mageed AB, Mondal D, Hellstrom WJ, et al. Differential expression of novel biomarkers (TLR-2, TLR-4, COX-2, and Nrf-2) of inflammation and oxidative stress in semen of leukocytospermia patients. Andrology. 2015; 3:848–855. PMID: 26227162.

96. Sikka SC, Toor JS, Lucelia D, Yafi FA, Hellstrom W. Measurement of oxidation-reduction potential (ORP) as a newer tool indicative of oxidative stress in infertile men with leukocytospermia. Poster presented at: 41st Annual America Society of Andrology Conference. 2016 Apr 2-5; New Orleans, LA, USA.
97. Kirby EW, Wiener LE, Rajanahally S, Crowell K, Coward RM. Undergoing varicocele repair before assisted reproduction improves pregnancy rate and live birth rate in azoospermic and oligospermic men with a varicocele: a systematic review and meta-analysis. Fertil Steril. 2016; 106:1338–1343. PMID: 27526630.

98. Majzoub A, Agarwal A. Antioxidant therapy in idiopathic oligoasthenoteratozoospermia. Indian J Urol. 2017; 33:207–214. PMID: 28717270.

99. Jung JH, Kim MH, Kim J, Baik SK, Koh SB, Park HJ, et al. Treatment of leukocytospermia in male infertility: a systematic review. World J Mens Health. 2016; 34:165–172. PMID: 28053945.

100. Wright C, Milne S, Leeson H. Sperm DNA damage caused by oxidative stress: modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod Biomed Online. 2014; 28:684–703. PMID: 24745838.

SUPPLEMENTARY MATERIALS
Scan this QR code to see the supplementary materials, or visit https://www.icurology.org/src/sm/icurology-58-385-s001.pdf.

Supplementary Table 1
Summary of the commonly used techniques to evaluate seminal oxidative stress
Supplementary Table 2
Semen parameters and oxidation-reduction potential (ORP) changes in all patients (n=28) who had repeated tests done over a period of 16.8±7.7 weeks
Supplementary Fig. 1
Correlation of semen static oxidation-reduction potential (sORP) (mV/106 sperm/mL) with semen parameters at 0 minutes in healthy controls (A, concentration; B, total sperm count; C, motility; D, morphology) and infertile patients (E, concentration; F, total sperm count; G, motility; H, morphology).
Supplementary Fig. 2
Correlation of seminal plasma static oxidation-reduction potential (sORP) (mV/106 sperm/mL) with semen parameters at 0 minutes in healthy controls (A, concentration; B, total sperm count; C, motility; D, morphology) and infertile patients (E, concentration; F, total sperm count; G, motility; H, morphology).
Supplementary Fig. 3
Correlation of semen static oxidation-reduction potential (sORP) (mV/106 sperm/mL) with semen parameters at 120 minutes in healthy controls (A, concentration; B, total sperm count; C, motility; D, morphology) and infertile patients (E, concentration; F, total sperm count; G, motility; H, morphology).
Supplementary Fig. 4
Correlation of seminal plasma static oxidation-reduction potential (sORP) (mV/106 sperm/mL) with semen parameters at 120 minutes in healthy controls (A, concentration; B, total sperm count; C, motility; D, morphology) and infertile patients (E, concentration; F, total sperm count; G, motility; H, morphology).
Supplementary Fig. 5
Receiver operating characteristic curve establishing the cutoff in semen (A) and seminal plasma (B). Distribution of static oxidation-reduction potential (sORP) (mV/106 sperm/mL) in subjects with normal and abnormal motility in semen (C) and seminal plasma (D), suggesting a criterion for sORP in distinguishing semen quality based on good (≥40%) and poor (≤40%) motility. AUC, area under the curve; NPV, negative predictive value; PPV, positive predictive value.
Supplementary Fig. 6
Distribution of static oxidation-reduction potential (sORP) (mV/106 sperm/mL) in (1) normal healthy controls; (2) controls with proven fertility; (3) controls with unproven fertility; (4) infertile patients; (5) infertile patients presenting with a clinical varicocele; and (6) those with idiopathic infertility.
Supplementary Fig. 7
Static oxidation-reduction potential (sORP) (mV/106 sperm/mL) across samples and observers showing (A) Intraobserver reliability by observing the replicate sORP measures for each of the three samples. Most replicates were similar to each other and across the 3 observers (01–03). (B) Interobserver reliability by comparing sORP-values across observers. The mean sORP for each observer was equivalent with similar standard error of the mean, suggesting that all observers obtained similar sORP-values for each of the 10 samples tested, which were measured in 4 replicates.
Supplementary Fig. 8
Receiver operating characteristic curve of oxidation-reduction potential (ORP) (mv/106 sperm/mL) in distinguishing infertile patients from healthy controls in the combined dataset (Cleveland Clinic and Doha, Qatar). Acc, accuracy; AUC, area under the curve; NPV, negative predictive value; PPV, positive predictive value; Sens, sensitivity; Spec, specificity.
Fig. 1
Schematic of the physiological/pathological roles of reactive oxygen species and sources leading to increased production.
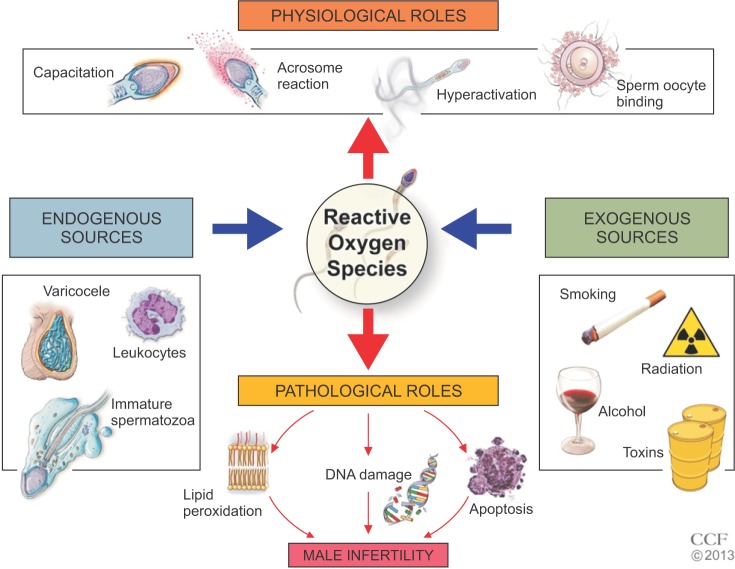
Fig. 3
A receiver operating characteristic curve was used to identify the static oxidation-reduction potential (mV/106 sperm/mL) criterion i.e., cutoff, sensitivity, and specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy and area under the curve (AUC) that best predicted the normal and abnormal semen parameters as well as differentiated normal healthy controls from male factor infertility patients.
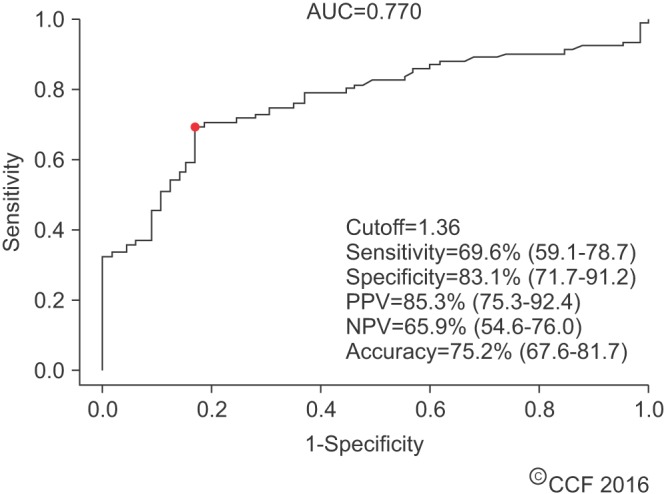
Fig. 4
Distribution of static oxidation-reduction potential (sORP) (mV/106 sperm/mL) in controls and patients showing the established cutoff values. Data are represented as box-plot showing median and interquartile range. The whiskers are the 95% confidence intervals.
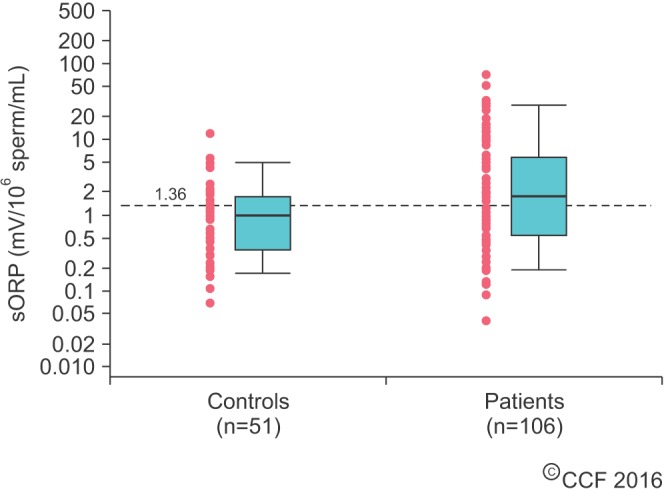
Fig. 5
Receiver operating characteristic curve of the oxidation-reduction potential (mV/106 sperm/mL) in different groups of abnormal sperm parameters: oligozoospermic group (A), asthenozoospermic group (B), and teratozoospermic group (C). Acc, accuracy; AUC, area under the curve; NPV, negative predictive value; PPV, positive predictive value; Sens, sensitivity; Spec, specificity.
Fig. 6
(A) Receiver operating characteristic curve of the oxidation-reduction potential (ORP) (mV/106 sperm/mL) in detecting at least 1 sperm parameter. (B) Box-and-whisker plots showing the distributions of the ORP cutoff between normal and abnormal sperm parameters. Acc, accuracy; AUC, area under the curve; NPV, negative predictive value; PPV, positive predictive value; Sens, sensitivity; Spec, specificity.
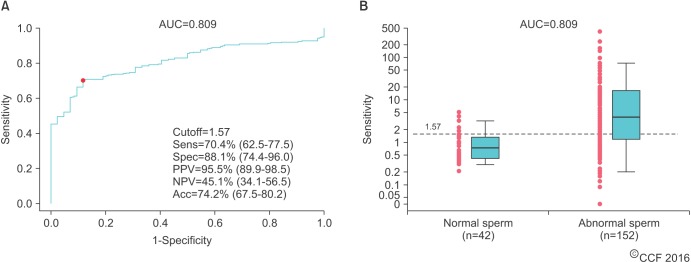
Table 1
List of key findings on ORP and male infertility in clinical studies
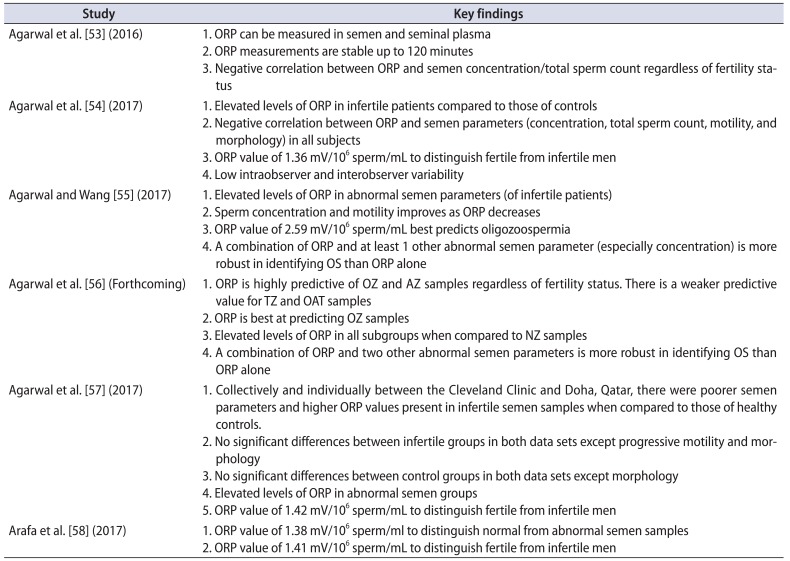
| Study | Key findings |
|---|---|
| Agarwal et al. [53] (2016) | 1. ORP can be measured in semen and seminal plasma |
| 2. ORP measurements are stable up to 120 minutes | |
| 3. Negative correlation between ORP and semen concentration/total sperm count regardless of fertility status | |
| Agarwal et al. [54] (2017) | 1. Elevated levels of ORP in infertile patients compared to those of controls |
| 2. Negative correlation between ORP and semen parameters (concentration, total sperm count, motility, and morphology) in all subjects | |
| 3. ORP value of 1.36 mV/106 sperm/mL to distinguish fertile from infertile men | |
| 4. Low intraobserver and interobserver variability | |
| Agarwal and Wang [55] (2017) | 1. Elevated levels of ORP in abnormal semen parameters (of infertile patients) |
| 2. Sperm concentration and motility improves as ORP decreases | |
| 3. ORP value of 2.59 mV/106 sperm/mL best predicts oligozoospermia | |
| 4. A combination of ORP and at least 1 other abnormal semen parameter (especially concentration) is more robust in identifying OS than ORP alone | |
| Agarwal et al. [56] (Forthcoming) | 1. ORP is highly predictive of OZ and AZ samples regardless of fertility status. There is a weaker predictive value for TZ and OAT samples |
| 2. ORP is best at predicting OZ samples | |
| 3. Elevated levels of ORP in all subgroups when compared to NZ samples | |
| 4. A combination of ORP and two other abnormal semen parameters is more robust in identifying OS than ORP alone | |
| Agarwal et al. [57] (2017) | 1. Collectively and individually between the Cleveland Clinic and Doha, Qatar, there were poorer semen parameters and higher ORP values present in infertile semen samples when compared to those of healthy controls. |
| 2. No significant differences between infertile groups in both data sets except progressive motility and morphology | |
| 3. No significant differences between control groups in both data sets except morphology | |
| 4. Elevated levels of ORP in abnormal semen groups | |
| 5. ORP value of 1.42 mV/106 sperm/mL to distinguish fertile from infertile men | |
| Arafa et al. [58] (2017) | 1. ORP value of 1.38 mV/106 sperm/ml to distinguish normal from abnormal semen samples |
| 2. ORP value of 1.41 mV/106 sperm/mL to distinguish fertile from infertile men |
Table 2
Semen parameters and oxidation-reduction potential (ORP) in subjects (n=547) with at least one abnormal semen parameter for combined dataset of Cleveland Clinic and Doha study populations
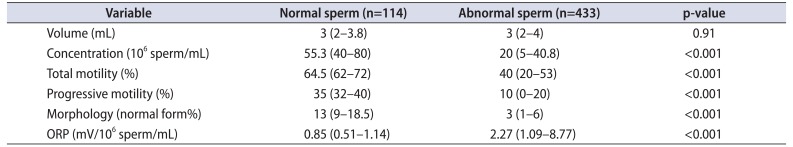
Values are presented as median (interquartile range).
Adapted from Agarwal et al. Andrology 2017;5:939-45 [57], permission of John Wiley and Sons.
Table 3
Semen parameters and oxidation-reduction potential (ORP) in infertile patients (n=497) with at least one abnormal semen parameter for combined dataset of Cleveland Clinic and Doha study populations
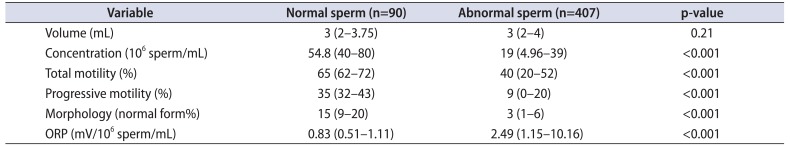
Values are presented as median (interquartile range).
Adapted from Agarwal et al. Andrology 2017;5:939-45 [57], permission of John Wiley and Sons.
Table 4
Predicting semen samples from infertile patients and from fertile donors
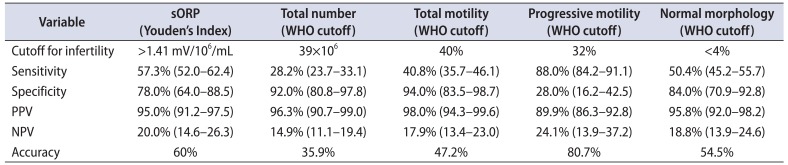
Values are presented with 95% confidence interval.
sORP, static oxidation-reduction potential; WHO, World Health Organization; PPV, positive predictive value; NPV, negative predictive value.
Adapted from Arafa et al. Andrologia 2017 Aug 3 [58], permission of John Wiley and Sons.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download