Abstract
Purpose
To study clinical presentation, laboratory results, imaging findings and treatment options and outcomes of retroperitoneal fibrosis (RPF). To determine whether it follows the same natural course and response to treatment in the Asian population as in the Western world.
Materials and Methods
Medical records of patients diagnosed with RPF on imaging and histopathology between February 2010 and April 2016 were reviewed.
Results
Of the 21 patients analyzed, mean age at presentation was 50.81 years. The male to female ratio was 0.9:1. Pain was most common presenting complaint (95.23% cases), almost 85% cases were idiopathic and rests were postradiation induced. The median creatinine level was 1.8 mg/dL. The mean erythrocyte sedimentation rate (ESR) was 53.2 mm/h. Hydronephrosis was present in all patients and 47.6% had atrophic kidneys. Diffuse retroperitoneal mass was present in 61.1%. Ureterolysis with lateralization, omental wrapping or gonadal pedicle wrap was done in 17 cases. Two patients underwent uretero-ureterostomy. One patient underwent ileal replacement of ureter, and one ileal conduit. Eighteen patients received concurrent medical treatment, 11 were given tamoxifen, 2 steroids (Prednisolone), and five were given both. Of the 20 patients with follow-up, 70% had complete symptomatic relief; ESR improvement was seen in 77.8%. Follow-up ultrasound showed resolved and decreased hydronephrosis in 20% and 55% respectively. One patient had treatment failure and 17.65% had disease recurrence.
Retroperitoneal fibrosis (RPF) is an uncommon but serious disorder characterized by chronic nonspecific inflammation of the retroperitoneum, which can entrap and obstruct retroperitoneal structures, notably the ureters [123]. RPF is a part of a class of disorders characterized by hyper proliferation of fibrous tissue in the retroperitoneum. The fibrotic process begins in the retroperitoneum just below the level of the renal arteries. Fibrosis gradually expands, encasing the ureters, inferior vena cava, aorta, mesenteric vessels, and/or sympathetic nerves.
The French Urologist Albarran is credited for first describing the surgical management of an extensive fibrotic process in the retroperitoneum of patients with ureteral obstruction in 1905. Later in 1948 Ormond [4] published his account of 2 cases in the English literature.
The idiopathic form accounts for more than two-thirds of all cases of RPF, with the remainder being secondary to different causes, such as tumors, infections, radiotherapy and drugs [5]. Smoking is a risk factor for RPF [6].
RPF begins with clinical symptoms of flank pain and unexplained systemic symptoms (fatigue, weight loss), and less frequently lower extremity swelling, oliguria or anuria and testicular pain. Serum markers of inflammation like erythrocyte sedimentation rate (ESR) and C-reactive protein are usually elevated. In the last few years, an association with IgG4 related disease has been shown and serum IgG4 levels are often elevated [7]. Because of the vague symptoms, diagnosis is often delayed. Diagnosis of RPF relies primarily on imaging studies. Histopathology is useful to rule out malignancy. Historically, treatment has focused on relieving the ureteral obstruction with cystoscopy assisted placement of ureteral stents followed by more definitive resolution of ureteric obstruction with open or laparoscopic ureterolysis. However, recently, medical measures - steroids and immunosuppressive agents are preferred. Currently, treatment in the acute phase rests on steroids with a tapering schedule, variably combined with immunosuppressive agents in the later period [8]. Surgical measures are reserved for patients refractory to medical management or those with advanced fibrosis requiring relief of ureteral obstruction.
The purpose of our study was to study clinical presentation, laboratory results, imaging findings and treatment offered and outcomes of RPF and to determine whether it follows the same natural course and response to treatment in Asian population as in the Western world.
We included patients diagnosed with RPF between February 2010 and April 2016 and treated under the Department of Urology, P.D. Hinduja National Hospital, Mumbai. Cases consisted of newly diagnosed and recurrent cases of RPF in the age group of 20 to 80 years. Only patients with histopathological confirmation were included.
Patients with active concurrent malignancy, pregnant females and those with ongoing radiation therapy were excluded from the study. We searched patients' medical records using the International Classification of Diseases, 10th edition.
Clinical history, physical examination and relevant laboratory investigations viz. complete hemogram, serum creatinine, and ESR were done. Serum IgG4 values were not available for our patients. Computed tomography (CT) scan was done at time of diagnosis and ultrasonography (USG) was done during follow-up, positron emission tomography (PET) scan was done in few patients.
Surgical intervention consisted of cystoscopy and stent placement in emergency setting for patients with hydronephrosis and renal insufficiency followed by definitive surgical correction in form of ureterolysis and lateralization or wrapping of ureters. Intraoperative biopsies were taken in all patients. Medical therapy in form of Tamoxifen and/or prednisone was received by patients for duration of 1 to 3 years postoperatively.
Outcome variables assessed at follow-up were symptomatic improvement, laboratory parameters improvement, radiological changes, recurrences, and treatment failures. Symptomatic improvement was defined as relief of pain without analgesics and systemic symptoms. Laboratory parameters improvement was defined as normalization of ESR to less than 30 mm/h, serum creatinine level less than 1.5 mg/dL or stable nonrising creatinine level. Radiological changes were monitored for hydronephrosis on USG and changes in the retroperitoneal mass on CT scan in few patients. Recurrence was defined as need for additional/secondary treatment after initial treatment success. Treatment failure was defined as persistence of symptoms and/or signs and/or laboratory parameters of active inflammation and/or deterioration in kidney function (serum creatinine) due to obstructive uropathy and /or worsening of radiological findings.
Quantitative and qualitative variables were compared pre- and posttreatment. For quantitative variables (serum creatinine, ESR) paired t-test or Wilcoxon signed rank test was conducted after performing normality testing. For all level of significance p-value was considered less than 0.05.
The study was approved by Institutional Review Board of P.D. Hinduja National Hospital & Medical Research Council (approval number: 934-15-SNS).
We obtained 26 patients record suffering from RPF from February 2010 to April 2016. Five patients were excluded as they did not fulfill the inclusion criteria. Three patients were excluded because of ongoing radiation treatment and two because of active concurrent malignancy.
In our study of 21 patients, the mean (range) age at presentation was 50.81 years (26–74 years) and 76.17% of patients were in the age group of 41 to 70 years. There were 10 male (47.62%) and 11 female patients (52.38%). The male to female ratio was 0.9:1. All 21 patients presented with either general systemic symptoms or organ specific symptoms (Table 1).
Three patients (14.3%) had received prior radiation therapy for carcinoma cervix. Immunological or associated medical disorders were present in 7 patients (33.3%) (Table 2).
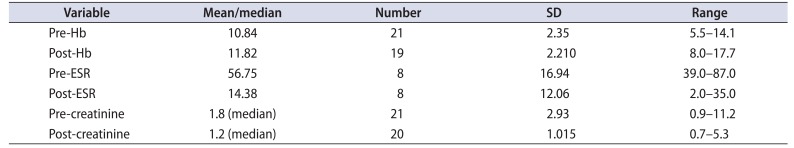
The mean (±standard deviation) hemoglobin at presentation was 10.84±2.4 g/dL. Acute/chronic renal insufficiency was present in 15 patients (71.43%). The median creatinine level was 1.8 mg/dL. ESR at presentation was available for 10 patients (47.6%), and was elevated (>30 mm in 1st hour) in all of them. The mean (±standard deviation) ESR was 53.2±16.71 mm in 1st hour.
Hydronephrosis of right kidney was present in 5 patients (23.81%), left kidney 6 patients (28.6%) and was bilateral in 10 patients (47.62%) on USG, intravenous urography or CT-scan (Fig. 1). Small kidneys were present in 10 patients (47.6%). Out of the 18 patients who underwent CT-scan or PET-CT scan at the time of presentation, 11 patients (61.1%) had a diffuse retroperitoneal mass encasing unilateral and/or bilateral ureters with or without the encasement of vessels (Fig. 2). Seven patients (38.9%) had periaortitis but no obvious diffuse retroperitoneal mass.
The details of the preoperative medical management were not available for 19 patients (90.4%); all these 19 patients had received steroids and/or tamoxifen elsewhere prior to being referred to our centre, details of duration for which were not available. Of the 2 patients for whom preoperative management details were available, one was treated with methotrexate for 5 months and 1 with Mycophenolate mofetil for 2 months before being referred to our institution. Methotrexate and mycophenolate mofetil were discontinued in these patients after the surgery.
Fifteen patients (71.43%) underwent double J-stenting for mean (range) 9.8 months (1.2–60 months), prior to any open surgical intervention. All 21 patients underwent ureterolysis at some stage in the management of their disease. Ureterolysis with ureteral lateralization with gonadal pedicle wrapping was done in 10 patients (47.6%), omental wrapping was done in 7 patients (33.3%). Two patients (9.5%) underwent uretero-ureterostomy, 1 for transection of ureter during the ureterolysis and other for nonviable short segment of ureter after ureterolysis. One patient underwent ileal replacement of ureter for nonviable long segment of mid and distal ureter. One patient underwent ileal conduit urinary diversion for postradiation shrunken bladder and vesico-vaginal fistula along with ureterolysis for postradiation RPF.
Eighteen patients received concurrent medical treatment, 11 (61.1%) were given tamoxifen, 2 (11.1%) were given steroids (Prednisolone), and 5 (27.8%) were given both. Three patients (14.3%) did not receive any medical treatment. The duration of postoperative medical management ranged from 1 to 3 years.
All patients had histologically proven RPF. Sixteen patients (76.2%) had idiopathic RPF, 3 patients (14.3%) had radiation induced RPF, and 2 patients (9.5%) had RPF with noncaseating granulomatous inflammation.
The median hospital stay was 7 days. Overall, postoperative complications were seen in 6 patients (28.6%). Three patients had minor wound discharge; 1 patient had urinary tract infection, 1 patient had prolonged paralytic ileus which delayed the hospital stay to 41 days. One patient who underwent ileal conduit for postradiation VVF had urine leak from anastomotic site and wound dehiscence, which required re-exploration and re-anastomosis, which healed satisfactorily.
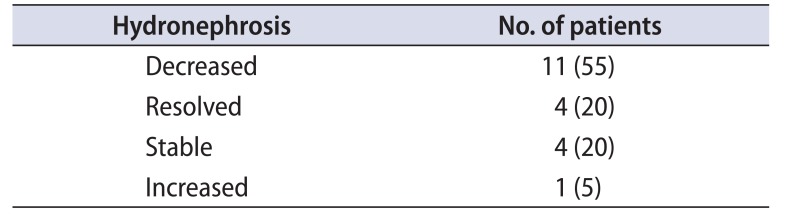
Follow-up was available for 20 patients (95.2%). The median follow-up duration was 1.7 years. Fourteen patients (70%) had complete symptomatic relief after treatment.
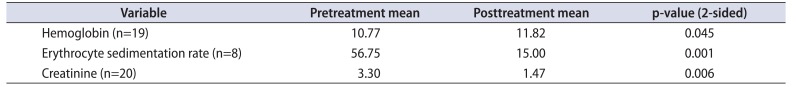
The pretreatment and posttreatment hemoglobin, ESR and serum creatinine results were analyzed (Table 3A, B). The paired t-test was used for comparing the pre-treatment and posttreatment ESR for eight patients and hemoglobin for 19 patients. The p-values for both the variables were 0.0003 and 0.045 respectively. The Wilcoxon Signed ranks test was applied to compare the pretreatment and posttreatment serum creatinine values for 20 patients. The p-value was 0.007, thus the test result was significant at p≤0.05. USG was available at follow-up for comparison with preoperative ultrasound (Table 4).
Recurrence of the disease was seen in 17.65% and required additional therapy, medical or resurgery. One patient had treatment failure and the disease progressed.
This was a retro-prospective study of patients diagnosed and treated for RPF. The mean age at diagnosis in our study was 50.81±12.23 years. The reported mean age at onset by Brandt et al. [6] in their study of 204 patients was between 50 to 60 years. The male to female ratio in our study was 0.9:1, which is in contrast with the available literature which states variation between 1:1 and 3:1 [15]. This could be because of different demographic pattern in India. As this is not a population based study, drawing conclusions regarding the gender distribution in RPF is difficult.
The symptoms and signs of RPF are those of systemic inflammatory disease and those resulting from compression of the affected retroperitoneal structures, notably the ureters. Pain was the most common presentation in our study population. Kermani et al. [9] found the most common presenting symptom as back pain (70 cases, 38%) and abdominal pain (73 cases, 40%). Bilateral lower limb edema was present in 19% of our patients and 2 patients (9.5%) had documented IVC thrombosis. Vascular complications are rarely described [101112], however, Brandt et al. [6] found such symptoms in more than 25% of patients.
History of smoking was present in 28.6% of our patients. This is less than the previous case series [13]. The less percentage of smoking in our patients can be attributed to more female patients and to the fact that none of them smoked.
Though our study showed elevated inflammatory markers in all patients, it is not unusual for patients with RPF to have normal markers of inflammation at diagnosis. In 2 other series, lower frequencies of between 10% and 51% have been reported [5]. The International Symposium on IgG4-related diseases, 2011 endorsed the consensus name of IgG4-related diseases and described the individual organ manifestations [14]. Since most of our patients were in the initial years after the recognition of IgG4-related RPF, serum IgG4 levels were not available for our cohort of patients.
Ureteral entrapment represents the hallmark of this disease and is the main reason for admittance in a urology department. Ureteral obstruction was present in all of our patients either unilateral or bilateral. Brandt et al. [6], in their study found ureteral obstruction in 95.6% patients which led to bilateral hydronephrosis in 55.9%. Hydronephrosis is reported in 47% to 100% in different case series [1516]. Hydronephrotic atrophy from unrecognized ureteral obstruction is a frequent complication of RPF. Koep and Zuidema [5] reported nephrectomy as the final surgical treatment of RPF in 8.1% of cases, assuming this was performed only in patients with atrophic kidney.
Histopathological diagnosis of RPF was obtained in all of our patients. The current imaging techniques cannot accurately differentiate accurately between idiopathic and malignant RPF, making a biopsy mandatory to confirm diagnosis and exclude underlying malignancy [171819].
The treatment of RPF has not been standardized till date [20]. Traditionally the approach to RPF has been surgical, but now after initial relief of urinary tract obstruction, medical strategies are used in most cases [121]. We treated our patients with a combination of surgical interventions and medications. The most frequently used protocol includes prednisolone administered 40–60 mg/d tapered to 10 mg/d in a time interval of 2–3 months followed by gradually discontinuation after 12–24 months. In 2002, van Bommel [11] conducted a study on 147 patients, which showed an improvement in 83% and a recurrence rate of 16% after discontinuation of corticosteroid therapy. Tamoxifen is used in different regimens in doses that vary between 10 mg to 40 mg per day for six months up to three years. Immunosuppressants have shown promising results in treatment of RPF, but were not adequately tried in our patients may be due the preference of our rheumatologist.
The purpose of surgical therapy is to resolve the ureteric obstruction and to exclude an underlying malignancy. Open ureterolysis with deep tissue biopsy, repositioning the ureters laterally, with intraperitonealization and/or omental wrapping are necessary in order to restore the renal function [12223]. Arvind et al. [24] concluded that laparoscopic ureterolysis and omental wrapping in the setting of obstructive uropathy were safe and effective alternative with a high success rate at mid to long term follow up. Mufarrij and Stifelman [25] reported the first robot-assisted laparoscopic ureterolysis with omental wrap.
In our study, nearly 70% had complete relief of their symptoms on follow-up. The decrease in the inflammatory marker (ESR) and serum creatinine values was statistically significant. As ESR was analyzed in less than 50 percent of our patients, it would not carry much importance. Kermani et al. [9] in their study showed that most patients did well, with stabilization (34%) or improvement (50%) in their renal function as evidenced by imaging studies.
Limitations of our study were that it was a single centre study. Sample size was small, hence generalization of our findings and statistical significance are limited. The relation between IgG4-related diseases and RPF could not be commented because of lack of IgG4 data. Our study could not evaluate the natural course of RPF in patients who improved and were cured by conservative treatment only. Despite being a fairly adequate follow-up, longer-term followup is mandatory, since recurrences have been reported after 10 years also.
RPF is a rare disease, with most cases being idiopathic. The male to female ratio may be equal in Asian population as against previous Western studies showing male predominance. Smoking could be a lesser contributing factor for RPF in Asian population compared to Western population. Renal dysfunction at presentation is not uncommon. Inflammatory markers are often significantly raised. Hydronephrosis is common complication of RPF. Biopsy is mandatory for confirmation of diagnosis. The initial goal of management is to relieve the obstruction and preserve renal function followed by medical management. The follow-up protocol should include the evaluation of local and systemic symptoms, assessment of renal function, analysis of acute phase reactants and evaluation of ureteral obstruction and the size of retroperitoneal mass on imaging. Long-term assessment is mandatory as recurrences have been reported even after 10 years. More clinical studies are needed to standardize the protocol for diagnosis, treatment and follow-up after medical or surgical management.
ACKNOWLEDGMENTS
The authors acknowledge The National Health and Education Society (the work was carried out at Hinduja Hospital, Mumbai).
References
1. Vaglio A, Salvarani C, Buzio C. Retroperitoneal fibrosis. Lancet. 2006; 367:241–251. PMID: 16427494.

2. van Bommel EF, Jansen I, Hendriksz TR, Aarnoudse AL. Idiopathic retroperitoneal fibrosis: prospective evaluation of incidence and clinicoradiologic presentation. Medicine (Baltimore). 2009; 88:193–201. PMID: 19593223.
3. Mitchinson MJ. Retroperitoneal fibrosis revisited. Arch Pathol Lab Med. 1986; 110:784–786. PMID: 3755888.

4. Ormond JK. Bilateral ureteral obstruction due to envelopment and compression by an inflammatory retroperitoneal process. J Urol. 1948; 59:1072–1079. PMID: 18858051.

5. Koep L, Zuidema GD. The clinical significance of retroperitoneal fibrosis. Surgery. 1977; 81:250–257. PMID: 841463.
6. Brandt AS, Kamper L, Kukuk S, Haage P, Roth S. Associated findings and complications of retroperitoneal fibrosis in 204 patients: results of a urological registry. J Urol. 2011; 185:526–531. PMID: 21168884.

7. Khosroshahi A, Stone JH. A clinical overview of IgG4-related systemic disease. Curr Opin Rheumatol. 2011; 23:57–66. PMID: 21124086.

8. Scheel PJ Jr, Feeley N. Retroperitoneal fibrosis. Rheum Dis Clin North Am. 2013; 39:365–381. PMID: 23597969.

9. Kermani TA, Crowson CS, Achenbach SJ, Luthra HS. Idiopathic retroperitoneal fibrosis: a retrospective review of clinical presentation, treatment, and outcomes. Mayo Clin Proc. 2011; 86:297–303. PMID: 21454732.

10. van Bommel EF, Hendriksz TR, Huiskes AW, Zeegers AG. Brief communication: tamoxifen therapy for nonmalignant retroperitoneal fibrosis. Ann Intern Med. 2006; 144:101–106. PMID: 16418409.

11. van Bommel EF. Retroperitoneal fibrosis. Neth J Med. 2002; 60:231–242. PMID: 12365466.
12. Littlejohn GO, Keystone EC. The association of retroperitoneal fibrosis with systemic vasculitis and HLA-B27: a case report and review of the literature. J Rheumatol. 1981; 8:665–669. PMID: 6975379.
13. Labidi J, Ariba YB, Chargui S, Bousetta N, Louzir B, Othmani S. Retroperitoneal fibrosis: a retrospective review of clinical presentation, treatment and outcomes. Saudi J Kidney Dis Transpl. 2015; 26:816–822. PMID: 26178567.

14. Stone JH, Khosroshahi A, Deshpande V, Chan JK, Heathcote JG, Aalberse R, et al. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum. 2012; 64:3061–3067. PMID: 22736240.

15. Marcolongo R, Tavolini IM, Laveder F, Busa M, Noventa F, Bassi P, et al. Immunosuppressive therapy for idiopathic retroperitoneal fibrosis: a retrospective analysis of 26 cases. Am J Med. 2004; 116:194–197. PMID: 14749165.

16. Saxton HM, Kilpatrick FR, Kinder CH, Lessof MH, McHardy-Young S, Wardle DF. Retroperitoneal fibrosis. A radiological and follow-up study of fourteen cases. Q J Med. 1969; 38:159–181. PMID: 5769557.
17. Kottra JJ, Dunnick NR. Retroperitoneal fibrosis. Radiol Clin North Am. 1996; 34:1259–1275. PMID: 8898793.
18. McCarthy JG, Porter MR, Veenema R. Retroperitoneal fibrosis and large bowel obstruction: case report and review of the literature. Ann Surg. 1972; 176:199–204. PMID: 5077079.
19. Drake MJ, Nixon PM, Crew JP. Drug-induced bladder and urinary disorders. Incidence, prevention and management. Drug Saf. 1998; 19:45–55. PMID: 9673857.
20. Drieskens O, Blockmans D, Van den Bruel A, Mortelmans L. Riedel's thyroiditis and retroperitoneal fibrosis in multifocal fibrosclerosis: positron emission tomographic findings. Clin Nucl Med. 2002; 27:413–415. PMID: 12045432.
21. Martorana D, Vaglio A, Greco P, Zanetti A, Moroni G, Salvarani C, et al. Chronic periaortitis and HLA-DRB1*03: another clue to an autoimmune origin. Arthritis Rheum. 2006; 55:126–130. PMID: 16463424.

22. Cronin GC, Lohan DG, Blake MA, Roche C, McCarthy P, Murphy JM. Retroperitoneal fibrosis: review of clinical features and imaging findings. Am J Roentgenol. 2008; 191:423–431. PMID: 18647912.
23. Heidenreich A, Derakhshani P, Neubauer S, Krug B. Treatment outcomes in primary and secondary retroperitoneal fibrosis. Urologe A. 2000; 39:141–148. PMID: 10768224.
24. Arvind NK, Singh O, Ali Q, Singh J, Gupta SS, Sahay S. Laparoscopic ureterolysis and omental wrapping in patients with retroperitoneal fibrosis and obstructive uropathy: a single-center experience. J Laparoendosc Adv Surg Tech A. 2014; 24:159–164. PMID: 24479819.

25. Mufarrij PW, Stifelman MD. Robotic ureterolysis, retroperitoneal biopsy, and omental wrap for the treatment of ureteral obstruction due to idiopathic retroperitoneal fibrosis. Rev Urol. 2006; 8:226–230. PMID: 17192802.
Fig. 1
Intravenous urography showing medialization of left ureter with hydroureteronephrosis and poorly functioning right kidney.
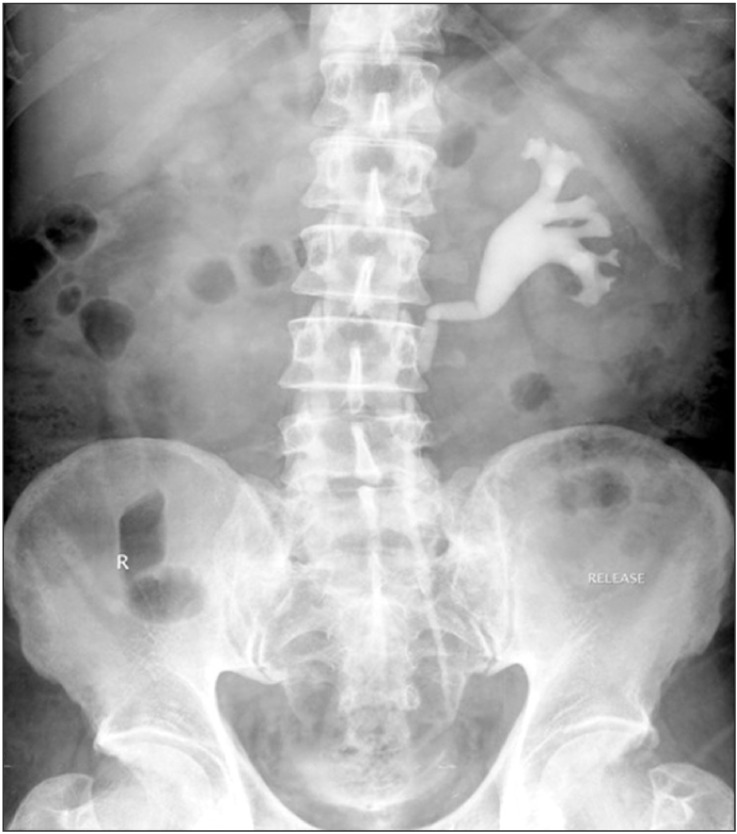
Fig. 2
Pretreatment (A) and posttreatment (B) computed tomography scan showing retroperitoneal mass and improvement in function and excretion on right side.
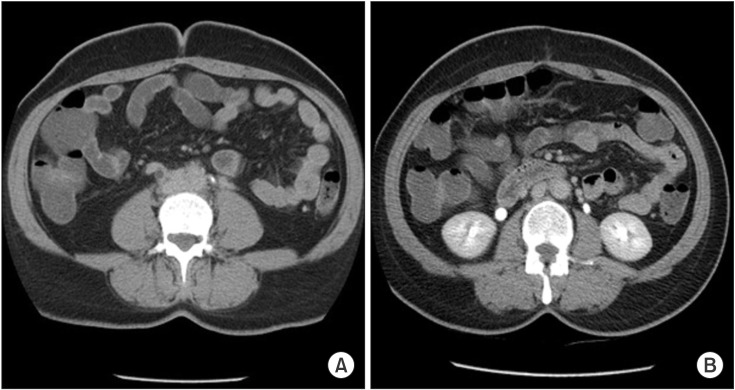
Table 1
Clinical presentation
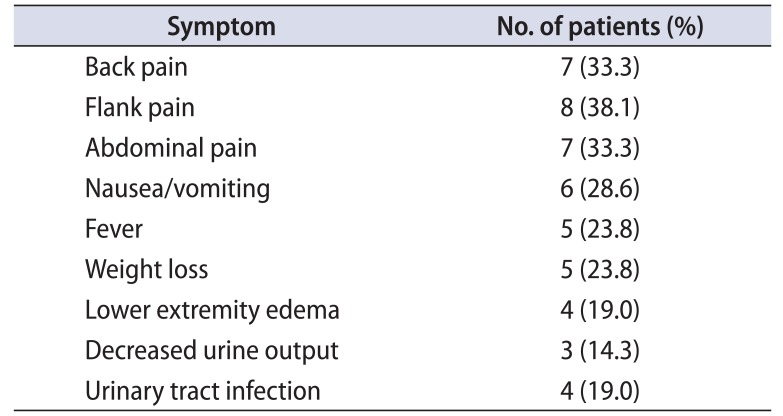
Table 2
Associated co morbidities
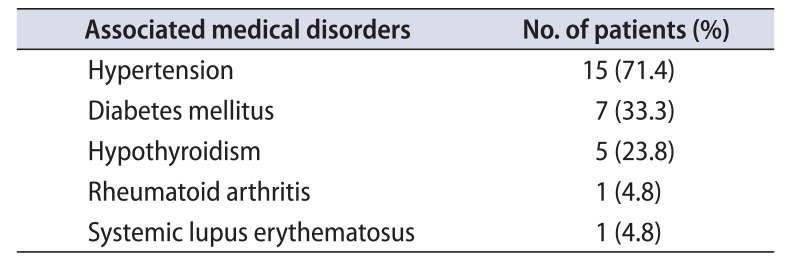
| Associated medical disorders | No. of patients (%) |
|---|---|
| Hypertension | 15 (71.4) |
| Diabetes mellitus | 7 (33.3) |
| Hypothyroidism | 5 (23.8) |
| Rheumatoid arthritis | 1 (4.8) |
| Systemic lupus erythematosus | 1 (4.8) |
Table 3A
Hemoglobin (Hb), erythrocyte sedimentation rate (ESR), and creatinine results




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download