Abstract
Purpose
To assess the prevalence of fluoroquinolone resistance among patients undergoing transrectal ultrasound (TRUS)-guided prostate biopsy and the impact of rectal swab culture-directed antibiotic prophylaxis on postbiopsy infectious complications.
Materials and Methods
We prospectively analyzed all patients undergoing TRUS-guided prostate biopsy from April 2013 to February 2015. Antibiotic prophylaxis was tailored to the results of rectal swab cultures. If the organism was fluoroquinolone-sensitive, oral ciprofloxacin 500 mg with tinidazole 600 mg was prescribed. If the organism was fluoroquinolone-resistant, then a culture-directed antibiotic was prescribed. In both cases the antibiotic was continued for 3 days. All patients were followed for 14 days after biopsy to record infectious complications.
Results
A total of 247 patients were included, and Escherichia coli was isolated on rectal swab cultures in 99.5% of the patients. Of these, 41.7% harbored fluoroquinolone-resistant E. coli. Piperacillin/tazobactam was the most common culture-directed antibiotic prescribed (59.3%), with amoxicillin/clavulanic being the second most common (25.5%) for the fluoroquinolone-resistant group. Only 2 patients (0.9%) developed postbiopsy fever and none had sepsis.
Transrectal ultrasound (TRUS)-guided prostate biopsy is the primary modality used to diagnose prostate cancer. It is a relatively safe procedure and is commonly performed on an outpatient basis. Most postprocedural complications are minor, such as hematuria, hematospermia, and hematochezia, and are self-limiting and therefore seldom require intervention. Of the major complications, infectious complications are the ones that are potentially life-threatening. Although uncommon (the incidence of urinary tract infections ranges from 2% to 6% and that of sepsis from 0.2% to 2%), postbiopsy infection is the most common reason for 30-d readmission and carries a significant cost burden [1234].
Escherichia coli is the most common causative organism implicated in 75% to 90% of cases of posttransrectal biopsy infections (PTRBIs) and is rectal in origin [156]. Routine administration of antibiotic prophylaxis results in a decline in PTRBIs from a range of 8%–87% to 3%–8.6%, leading most associations to recommend fluoroquinolone as the agent of choice for antimicrobial prophylaxis for prostate biopsy [7]. With their high concentration in the prostate and good activity against gram-negative bacteria, fluoroquinolones have been shown to reduce PTRBI from 25% to 8% [89].
Fluoroquinolone is the current prophylaxis of choice for most urologic procedures. As a result, its widespread use has led to colonization of rectal flora with fluoroquinolone-resistant E. coli in 22.8% of patients undergoing prostate biopsy [10]. Also as a result, the number of PTRBIs has been reported to rise from 0.52 to 2.15 infections per 100 biopsies in the past few years, and most cases of PTRBI or sepsis in the recent literature are caused by fluoroquinolone-resistant E. coli [5]. Because PTRBIs are caused by rectal flora, rectal swab culture-directed antibiotic prophylaxis may be the most reasonable approach in reducing infections. The goal of this study was to assess the prevalence of fluoroquinolone resistance among patients undergoing TRUS-guided prostate biopsy and the impact of rectal swab culture-directed antibiotic prophylaxis on PTRBI complications.
After Institutional Review Board approval (Institutional Ethical Sub Committee [IESC]/T-68/01.02.2013), we prospectively analyzed patients undergoing TRUS-guided prostate biopsy at a tertiary care center from April 2013 to February 2015. All patients undergoing TRUS-guided prostate biopsy for suspected prostatic carcinoma either on the basis of an abnormal rectal examination result or raised serum prostate-specific antigen values with no prior history of prostate biopsy were included. All patients had prebiopsy urine cultures. If the results of the prebiopsy urine culture were positive, culture-directed antibiotics were given and biopsy was performed only after documenting a sterile urine culture. Rectal swab cultures of all patients were taken 1 week prior to biopsy. Rectal swabs obtained from the patients were inoculated into 2 MacConkey agar plates, one plain and other containing ciprofloxacin (10 µg/mL). The plain MacConkey agar plate identified the dominant rectal flora and acted as a quality control to ensure specimen adequacy. If there was growth on plain MacConkey agar but not on ciprofloxacin-containing MacConkey agar, the organism was categorized as fluoroquinolone-sensitive. If there was growth on ciprofloxacin-containing MacConkey agar, the organism was categorized as fluoroquinolone-resistant. Fluoroquinolone-resistant organisms were then subcultured and a standard bacteriological method was used to determine the antibiotic sensitivity pattern (Kirby Bauer Method) of the isolated organism.
All patients underwent TRUS-guided 12-core needle prostate biopsy. As a part of the prebiopsy preparation, routine antibiotic prophylaxis and rectal cleansing with an enema was used. If the organism grown was fluoroquinolone-sensitive, then a standard empirical antibiotic regimen was prescribed. It comprised oral ciprofloxacin 500 mg along with tinidazole 600 mg the evening before and on the morning of the biopsy and was continued for 3 days after biopsy as a twice daily dose in the same strength. If the organism was fluoroquinolone-resistant, then a culture-directed antibiotic was given the night before and on the morning of the biopsy and continued for 3 days. All patients received a proctoclysis enema on the morning of the biopsy. After biopsy all patients were followed for a period of 14 days and infectious complications such as fever >38.5℃, urinary tract infection, epididymo-orchitis, prostatitis, pyelonephritis, and sepsis were recorded.
A total of 247 patients were included in this study. Rectal swabs were assessed for all of them. In all except one, E. coli was isolated on rectal swab cultures (99.5%), making it the most common organism isolated. Of these cultures, 41.7% (103 patients) harbored fluoroquinolone-resistant E. coli and most of these cultures were sensitive to piperacillin/tazobactam (85.4%). Table 1 summarizes the organisms isolated, their fluoroquinolone sensitivity, and the antibiograms of the fluoroquinolone-resistant organisms.
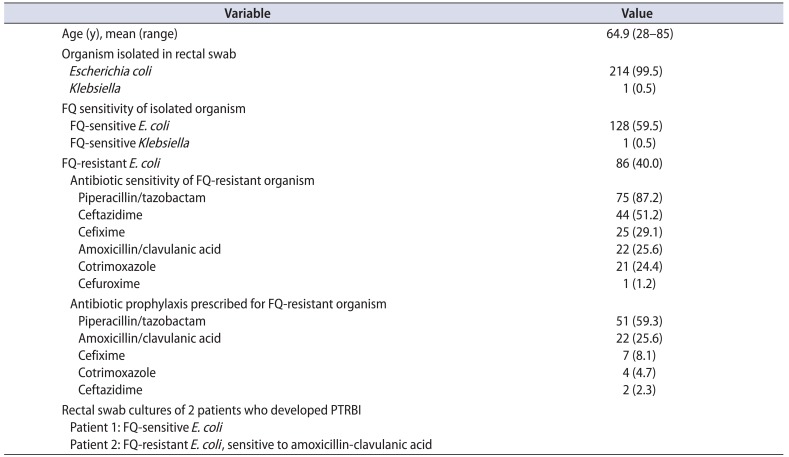
Of the 247 patients, 87.0% (215 patients) underwent transrectal prostate biopsy with the described method of prebiopsy preparation and 13% (32 patients) did not (10 lost to follow-up, 8 refused biopsy, and 4 repeated serum prostate specific antigen, which was normal). Of those who underwent transrectal prostate biopsy, 40% (86 of 215 patients) had colonization with fluoroquinolone-resistant organisms and culture-directed antibiotics were given in all cases. Oral antibiotics were preferred over intravenous whenever possible. The most common antibiotic used was piperacillin/tazobactam, to which 87% of the fluoroquinolone-resistant organisms were sensitive. Table 2 summarizes the organisms isolated, the fluoroquinolone sensitivity, the antibiograms of the fluoroquinolone-resistant organisms, and the antibiotics used in the fluoroquinolone-resistant group.
Out of those who underwent biopsy, only 2 patients (0.9%) developed postbiopsy fever, which subsided with conservative treatment and did not require admission. None of the patients had sepsis or required intervention. The first patient who developed fever had diabetes for the past 8 years, was self-voiding, and had a sterile urine culture. The prebiopsy rectal swab cultured E. coli sensitive to fluoroquinolone and the patient was prescribed the combination of oral ciprofloxacin and tinidazole. He had one spike of fever on day 1 after biopsy which did not recur and he was continued on the same medications. The second patient had an indwelling catheter with mixed growth on urine culture that became sterile after a short course of nitrofurantoin. His rectal swab cultured E. coli was resistant to fluoroquinolone and he was given an amoxicillin-clavulanic acid combination as per sensitivity. He also had fever on days 1 and 2, which was low grade and resolved with the same medications. None of these cases required admission or a change in antibiotics.
Current literature indicates rising rates of PTRBI, which has been attributed to rampant colonization of the rectum by fluoroquinolone-resistant E. coli [5]. In the current study, the most common organism isolated was E. coli, which was fluoroquinolone-resistant in 40% of patients. The antibiogram of fluoroquinolone-resistant organisms revealed that most were sensitive to either piperacillin/tazobactam or amoxicillin/clavulanic acid, making them the most common antibiotics used in our study. With this protocol, we were able to achieve very low (0.9%) rates of PTRBI. Thus, culture-directed antibiotic prophylaxis for prostate biopsy could reduce infectious complications to a very low level despite exceedingly high fluoroquinolone resistance rates at our center.
Current recommendations on antibiotic prophylaxis before prostate biopsy specify the use of fluoroquinolone, but the duration of prophylaxis is debatable. Some prefer only a single dose, whereas others use a 3-d or a 5-d regimen [1112]. A randomized controlled trial showed that antibiotic prophylaxis reduces postbiopsy complications versus a placebo but showed no difference between a single dose and a 3-d regimen [11]. Currently, fluoroquinolone has become standard antibiotic prophylaxis for most urologic procedures, leading to its wide and indiscriminate use in outpatient settings [13]. Any patient who has received fluoroquinolone in the preceding months has a high likelihood of harboring fluoroquinolone-resistant organisms in their fecal flora and thus being at higher risk of PTRBI if fluoroquinolone is prescribed again [14]. Furthermore, this resistance persists for months or even years after fluoroquinolone exposure. Thus, even if the organism is initially sensitive, it will inevitably become resistant if repeat biopsy or intervention is required [1516].
Most cases of PTRBI or sepsis in the recent literature are caused by fluoroquinolone-resistant E. coli. Batura et al. [6] assessed prebiopsy rectal swab cultures and found that 10.6% patients harbored fluoroquinolone-resistant organisms. Furthermore, they showed that all except 1 case of postbiopsy urinary tract infection or sepsis were caused by fluoroquinolone-resistant E. coli, which suggests a strong correlation between rectal swab isolates and organisms causing infectious complications. Similarly, another study reported a 19.6% prevalence of fluoroquinolone-resistant colonization, and 7 of 9 cases of PTRBI were caused by fluoroquinolone-resistant organisms [17]. In the current study, 40% of patients undergoing prostate biopsy harbored fluoroquinolone-resistant bacteria. Such a high rate of antibiotic resistance may be attributed to indiscriminate use of fluoroquinolone leading to the development of resistance. Thus, the rising rates of PTRBI in recent years are attributed to rising colonization of fecal flora by fluoroquinolone-resistant organisms [5]. We used MacConkey agar with 10 µg/mL of ciprofloxacin as the culture media in the present study, and at this high concentration, bacteria with moderate and low sensitivity to ciprofloxacin will also be inhibited and labeled as ciprofloxacin-sensitive. These patients were given ciprofloxacin for prebiopsy prophylaxis despite low sensitivity, and this might have resulted in higher chances of PTRBI. Despite this, however, only one patient in the current study had PTRBI with a fluoroquinolone-sensitive organism. The benefit of use of MacConkey agar with ciprofloxacin at a concentration of 1 µg/mL needs to be defined further.
Three strategies have been proposed to counter the increasing rates of PTRBI due to fluoroquinolone-resistant organisms. First is the targeted approach, for which prebiopsy rectal swab culture-directed antibiotic prophylaxis has been shown to reduce PTRBI rates [1718]. Taylor et al. [17] showed that with the use of a targeted approach, infectious complication rates were reduced from 2.6% to 0%. Although not statistically significant, they did not note any infectious complications in the targeted prophylaxis arm of 112 patients; whereas 9 (including 1 case of sepsis) of 345 patients had postbiopsy infection in the empirical group. Similarly, Duplessis et al. [18] compared 235 patients in a targeted group with 103 historical controls forming the empirical prophylaxis group and showed that PTRBI was reduced from 2.9% to 0% with targeted antimicrobial prophylaxis. A recent review of the literature noted a reduction in PTRBI rates from 4.5% with empirical prophylaxis to 0.7% with targeted prophylaxis and a reduction in sepsis rates from 2.2% to 0.4% with targeted prophylaxis (p<0.001), thus favoring targeted prophylaxis [10]. We also observed similar low rates of PTRBI (0.9%) with the use of a targeted approach. Our study did not have a control arm, but comparison of our PTRBI rates with those reported in the literature with empirical prophylaxis suggests that targeted prophylaxis reduces postbiopsy infections. This reduction will be more significant in our setting because of the high rates of fluoroquinolone resistance in the population under study.
A second approach to counter the increasing rates of PTRBI is an augmented prophylaxis approach, in which another broad-spectrum antibiotic is prescribed in addition to a standard fluoroquinolone regimen. Augmented prophylaxis has been shown to reduce infectious complications and is comparable to the targeted approach [1920]. The commonly used antibiotics in the augmented approach are fluoroquinolone with gentamycin, ceftriaxone, amikacin, or even imipenem [20]. The main drawback of the augmented approach is the use of multiple broad-spectrum antibiotics, which is actually an overtreatment for organisms that are sensitive to fluoroquinolone and increases the risk of development of multidrug-resistant strains. Despite the use of multiple drugs, there may be a few cases in which the organism is resistant to all antibiotics administered, thus risking PTRBI.
The third approach is prophylactic rectal cleansing with povidone-iodine, which mostly shows no significant benefit over control [21]. Thus, the targeted approach, which avoids overtreatment and the development of multidrug resistance, is the approach favored by most.
Targeted prophylaxis uses culture-directed antibiotics; thus, the choice of antibiotic for prophylaxis is determined by the local sensitivity patterns of each caregiving facility as well as by physician preference. Trimethoprim/sulfamethoxazole or gentamycin are the most commonly used antibiotics, either as a targeted approach or as part of an augmented approach [22]. Seldom, higher injectable antibiotics such as imipenem or aztreonam are used [20]. We preferably used amoxicillin/clavulanic acid over other oral antibiotics, and if the organism was resistant to all tested oral antibiotics, piperacillin/tazobactam was utilized. The use of broader spectrum antibiotics may explain the low rate of infectious complications in the current study despite a very high rate of fluoroquinolone resistance, although this would have resulted in higher chances of developing resistance.
The main drawback of a targeted approach in a developing country is that it is resource-intensive and requires rectal swab cultures of all patients with the associated cost. But by reducing the chances of PTRBI and its subsequent treatment and by reducing the development of multidrug-resistant strains as compared to the augmented approach, a targeted approach actually reduces overall health care costs. Taylor et al. [17] showed that to prevent one PTRBI, 38 patients need to undergo prebiopsy rectal swabs and that averting one PTRBI results in cost saving of US $4,499. They concluded that, overall, targeted prophylaxis is cost-effective. Furthermore, if cost is an issue and rectal swabs cannot be cultured in all patients, then those with prior exposure to fluoroquinolone or those at higher risk of PTRBI, such as patients with diabetes, chronic pulmonary disease, or an indwelling catheter, must receive targeted prophylaxis [5]. To conclude, culture-directed prophylaxis reduces the rates of PTRBI.
Fluoroquinolone-resistant bacteria are found in 40% of rectal swab cultures of men undergoing prostate biopsy. Use of targeted antibiotic prophylaxis results in low rates of PTRBI. Targeted prophylaxis using the results of prebiopsy rectal swab cultures may be a means of reducing the chance of PTRBI.
References
1. Williamson DA, Barrett LK, Rogers BA, Freeman JT, Hadway P, Paterson DL. Infectious complications following transrectal ultrasound-guided prostate biopsy: new challenges in the era of multidrug-resistant Escherichia coli. Clin Infect Dis. 2013; 57:267–274. PMID: 23532481.

2. Pinkhasov GI, Lin YK, Palmerola R, Smith P, Mahon F, Kaag MG, et al. Complications following prostate needle biopsy requiring hospital admission or emergency department visits - experience from 1000 consecutive cases. BJU Int. 2012; 110:369–374. PMID: 22313996.

3. Young JL, Liss MA, Szabo RJ. Sepsis due to fluoroquinolone-resistant Escherichia coli after transrectal ultrasound-guided prostate needle biopsy. Urology. 2009; 74:332–338. PMID: 19464041.

4. Lange D, Zappavigna C, Hamidizadeh R, Goldenberg SL, Paterson RF, Chew BH. Bacterial sepsis after prostate biopsy--a new perspective. Urology. 2009; 74:1200–1205. PMID: 19815258.

5. Carignan A, Roussy JF, Lapointe V, Valiquette L, Sabbagh R, Pépin J. Increasing risk of infectious complications after transrectal ultrasound-guided prostate biopsies: time to reassess antimicrobial prophylaxis? Eur Urol. 2012; 62:453–459. PMID: 22575912.

6. Batura D, Rao GG, Nielsen PB. Prevalence of antimicrobial resistance in intestinal flora of patients undergoing prostatic biopsy: implications for prophylaxis and treatment of infections after biopsy. BJU Int. 2010; 106:1017–1020. PMID: 20346055.

7. Liss MA. Infection: prostate biopsy-infection and prior fluoroquinolone exposure. Nat Rev Urol. 2011; 8:592–594. PMID: 21989304.
8. Kapoor DA, Klimberg IW, Malek GH, Wegenke JD, Cox CE, Patterson AL, et al. Single-dose oral ciprofloxacin versus placebo for prophylaxis during transrectal prostate biopsy. Urology. 1998; 52:552–558. PMID: 9763070.

9. Zani EL, Clark OA, Rodrigues Netto. Antibiotic prophylaxis for transrectal prostate biopsy. Cochrane Database Syst Rev. 2011; (5):CD006576. PMID: 21563156.

10. Cussans A, Somani BK, Basarab A, Dudderidge TJ. The role of targeted prophylactic antimicrobial therapy before transrectal ultrasonography-guided prostate biopsy in reducing infection rates: a systematic review. BJU Int. 2016; 117:725–731. PMID: 26709240.

11. Sabbagh R, McCormack M, Péloquin F, Faucher R, Perreault JP, Perrotte P, et al. A prospective randomized trial of 1-day versus 3-day antibiotic prophylaxis for transrectal ultrasound guided prostate biopsy. Can J Urol. 2004; 11:2216–2219. PMID: 15182413.
12. Wolf JS Jr, Bennett CJ, Dmochowski RR, Hollenbeck BK, Pearle MS, Schaeffer AJ, et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol. 2008; 179:1379–1390. PMID: 18280509.

13. Bootsma AM, Laguna Pes MP, Geerlings SE, Goossens A. Antibiotic prophylaxis in urologic procedures: a systematic review. Eur Urol. 2008; 54:1270–1286. PMID: 18423974.

14. Simsir A, Kismali E, Mammadov R, Gunaydin G, Cal C. Is it possible to predict sepsis, the most serious complication in prostate biopsy? Urol Int. 2010; 84:395–399. PMID: 20224265.

15. Loeb S, Vellekoop A, Ahmed HU, Catto J, Emberton M, Nam R, et al. Systematic review of complications of prostate biopsy. Eur Urol. 2013; 64:876–892. PMID: 23787356.

16. Aron M, Rajeev TP, Gupta NP. Antibiotic prophylaxis for transrectal needle biopsy of the prostate: a randomized controlled study. BJU Int. 2000; 85:682–685. PMID: 10759665.

17. Taylor AK, Zembower TR, Nadler RB, Scheetz MH, Cashy JP, Bowen D, et al. Targeted antimicrobial prophylaxis using rectal swab cultures in men undergoing transrectal ultrasound guided prostate biopsy is associated with reduced incidence of postoperative infectious complications and cost of care. J Urol. 2012; 187:1275–1279. PMID: 22341272.

18. Duplessis CA, Bavaro M, Simons MP, Marguet C, Santomauro M, Auge B, et al. Rectal cultures before transrectal ultrasound-guided prostate biopsy reduce post-prostatic biopsy infection rates. Urology. 2012; 79:556–561. PMID: 22386395.

19. Adibi M, Hornberger B, Bhat D, Raj G, Roehrborn CG, Lotan Y. Reduction in hospital admission rates due to post-prostate biopsy infections after augmenting standard antibiotic prophylaxis. J Urol. 2013; 189:535–540. PMID: 22982426.

20. Liss MA, Kim W, Moskowitz D, Szabo RJ. Comparative effectiveness of targeted vs empirical antibiotic prophylaxis to prevent sepsis from transrectal prostate biopsy: a retrospective analysis. J Urol. 2015; 194:397–402. PMID: 25846415.

21. Abughosh Z, Margolick J, Goldenberg SL, Taylor SA, Afshar K, Bell R, et al. A prospective randomized trial of povidone-iodine prophylactic cleansing of the rectum before transrectal ultrasound guided prostate biopsy. J Urol. 2013; 189:1326–1331. PMID: 23041343.

22. Dai J, Leone A, Mermel L, Hwang K, Pareek G, Schiff S, et al. Rectal swab culture-directed antimicrobial prophylaxis for prostate biopsy and risk of postprocedure infection: a cohort study. Urology. 2015; 85:8–14. PMID: 25458193.

Table 1
Results of rectal swab cultures of patients included in the study (n=247)
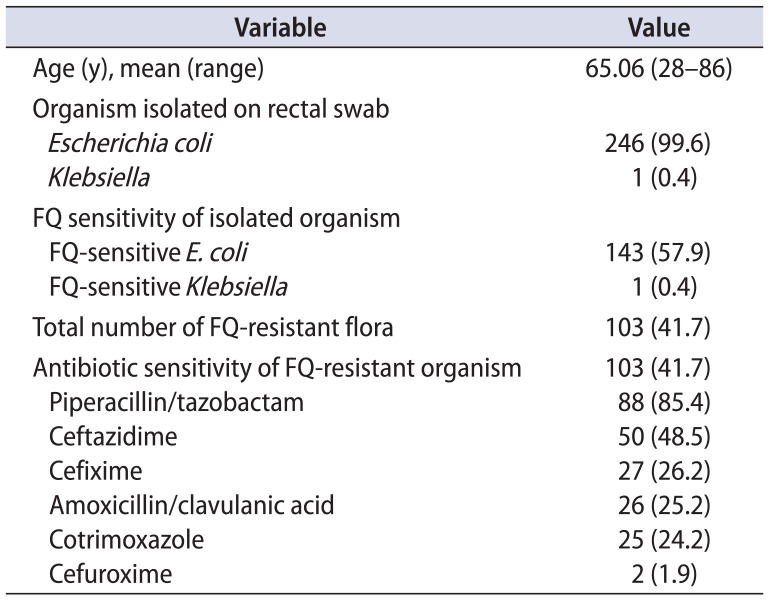
Table 2
Characteristics of the patients who underwent prostate biopsy (n=215)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download