Abstract
The da Vinci robotic system has improved surgeon dexterity, ergonomics, and visualization to allow for a minimally invasive option for complex reconstructive procedures in children. Over the past decade, robot-assisted laparoscopic ureteral reimplantation (RALUR) has become a viable minimally invasive surgical option for pediatric vesicoureteral reflux (VUR). However, higher-than-expected complication rates and suboptimal reflux resolution rates at some centers have also been reported. The heterogeneity of surgical outcomes may arise from the inherent and underestimated complexity of the RALUR procedure that may justify its reclassification as a complex reconstructive procedure and especially for robotic surgeons early in their learning curve. Currently, no consensus exists on the role of RALUR for the surgical management of VUR. High success rates and low major complication rates are the expected norm for the current gold standard surgical option of open ureteral reimplantation. Similar to how robot-assisted laparoscopic surgery has gradually replaced open surgery as the most utilized option for prostatectomy in prostate cancer patients, RALUR may become a higher utilized surgical option in children with VUR if the adoption of standardized surgical techniques that have been associated with optimal outcomes can be adopted during the second decade of RALUR. A future standard of RALUR for children with VUR whose parents seek a minimally invasive surgical option can arise if widespread achievement of high success rates and low major complication rates can be obtained, similar to the replacement of open surgery with robot-assisted laparoscopic radical prostectomy as the new strandard for men with prostate cancer.
Vesicoureteral reflux (VUR) in children has been referred as the “prostate cancer” of pediatric urology, as the 2 conditions share many features such as the high prevalence rates in their respective populations, the recent decline in utilization of diagnostic studies for these conditions, the historically high utilization of surgical intervention in the 2 conditions, as well as other features. VUR has a relatively high prevalence in children, where it occurs in approximately 1% of the general pediatric population [1], similar to the high prevalence of prostate cancer in men, one of the most common cancers in the world [2]. In addition, the screening or diagnostic tests for both diseases also appear to be in decline due to recently released guidelines. The updated American Academy of Pediatrics (AAP) guidelines [3] on the diagnosis and management of urinary tract infections (UTIs) in febrile infants and young children has led to the delay of voiding cystourethrography (VCUG) until the second UTI, which has decreased the diagnostic incidence of VUR [4]. Similarly, in 2012, the U.S. Preventive Services Task Force recommended against prostate-specific antigen (PSA) screening for prostate cancer, as they concluded that the potential benefits of screening were not greater than the expected risks; however, the debate on PSA screening is still continuing [5]. Furthermore, the historically high utilization of surgical intervention in the 2 conditions has led to the introduction of minimally invasive surgical options for both conditions. Robot-assisted laparoscopic radical prostatectomy has already become the new standard surgical treatment of prostate cancer for 90% of current prostate cancer surgical candidates as a result of its favorable and enduring oncologic outcomes comparable to open surgery and the relatively low complication rates for erectile dysfunction and postprostatectomy urinary incontinence [6].
Indications for surgical treatment of pediatric VUR include breakthrough UTI while on continuous antibiotics prophylaxis, renal scarring, and worsening or unresolving VUR. Determining which approach is most effective for treating a particular pediatric patient with VUR involves weighing the pros and cons of various antireflux surgeries with respect to success rates, durability of results, avoidance of febrile UTIs, universal applicability, general availability, perioperative morbidity, wound cosmesis, and cost [7]. Traditionally, open ureteral reimplantation is considered the gold standard surgical intervention for VUR [8]. However, recently, minimally invasive techniques such as robot-assisted laparoscopic surgery has been increasingly utilized for the treatment of pediatric surgical conditions [910], as robotic surgical systems have provided surgeons with 3-dimensional vision, 10x magnification, and fully articulating endo-wrists suitable for dissection and suturing. As a result, minimally invasive surgery options have become increasingly available for many complex reconstructive procedures in pediatric urology. Since first introduced in 2004 [11], robot-assisted laparoscopic ureteral reimplantation (RALUR) has been described as one of these new minimally invasive procedures that have been increasingly adopted and described in several clinical series [1213141516171819202122232425262728293031].
In this article, we reviewed the literature over the past decade on pediatric RALUR with a critical analysis of the perioperative outcomes with respect to reflux resolution and complications. As a result of this review, based upon this analysis, we provide tips and tricks on how to facilitate the best possible outcomes with RALUR to enable this minimally invasive surgical option to become a new standard treatment option for VUR in children similar to the evolution of radical prostatectomy from an open procedure to become the new standard surgical treatment option for prostate cancer in adult men.
RALUR has become more prevalent over the past decade but still remains relatively uncommon in many pediatric centers. Bowen et al. [32] recently analyzed the use of pediatric open, laparoscopic and RALUR in the United States from 2002 to 2012. The number of annual ureteral reimplantations decreased by 14.3% during this time period, but the minimally invasive ureteral reimplantations increased from 0.3% in 2000 to 6.3% in 2012. Of the minimally invasive ureteral reimplantations, 81.2% were robot-assisted laparoscopic.
While robotic surgery has increased in volume over the past decade in the field of pediatric urology, RALUR has lagged behind the increase of robot-assisted laparoscopic pyeloplasty (RALP). In our personal surgical experience (unpublished data), the number of RALUR cases was actually the highest in case volume as of 2010, while RALP was second highest as a result of the high prevalence of VUR in the pediatric population. However, the revised 2011 AAP guideline for the workup of pediatric UTI, where VCUG was not recommended routinely after the first febrile UTI led to decreases in the number of newly diagnosed VUR patients and the subsequent number of ureteral reimplantation surgeries. Of note, though, RALUR still remains a relatively high volume procedure for a pediatric robotic surgery program (Fig. 1). Another factor for why robotic ureteral reimplantation has not enjoyed as widespread adoption as quickly as robotic prostatectomy may be related to the necessary learning curve for the RALUR procedure. While many centers have reported favorable outcomes and safety profiles for RALUR [13151826], other centers have reported outcomes that did not reach the levels associated with traditional open reimplantation, indicating that the procedure's complexity may have been underestimated [2527]. A potential remedy for this is for surgeons early in their learning curve for robotic surgery to defer RALUR procedures until a later point in their robotic experience.
The intravesical approach of RALUR is composed of the following surgical steps: creation of pneumovesicum by the instillation of CO2 gas into the bladder, intravesical ureteral mobilization, submucosal tunneling, and intravesical advancement of the mobilized ureters within the newly created submucosal tunnels. This requires meticulous dissection and suturing in a limited working space. Since Peters and Woo [12] first described this procedure using a cross-trigonal or Cohen method, only 2 other series have reported their experiences with intravesical RALUR (Table 1) [121617].
Peters and Woo [12] reported their clinical series of 6 children 5 to 15 years old without the need for open conversion. Five of 6 patients (83%) had radiographic resolution of the VUR, while 1 patient had a urine leak postoperatively secondary to inadequate bladder closure. In 2011, Marchini et al. [16] reported on their series of patients who underwent intravesical RALUR (n=19) with intravesical open ureteral reimplantation (n=22), as well as extravesical RALUR (n=20) with extravesical open ureteral reimplantation (n=17). In their series, intravesical RALUR was associated with shorter durations of urinary catheter drainage, fewer bladder spasms, and shorter hospital stays when compared to the open intravesical technique, but with a higher reported complication rate. In 2012, Chan et al. [17] reported their intravesical RALUR experience in three children, all with high-grade bilateral VUR. All 3 patients (100%) showed reflux resolution on postoperative VCUG, and were free from UTI during the follow-up period.
A notable point is that, despite the similar time of introduction of intravesical RALUR in the literature with conventional laparoscopic intravesical ureteral reimplantation [33], no other reports on intravesical RALUR have been published in the literature beyond the initial articles unlike the increasing number of articles of further procedural development with conventional laparoscopic intravesical ureteral reimplantation [34353637383940]. Technical challenges with laparoscopic reimplantation and the expansion of the number of robotic systems worldwide may partially explain this evolution toward robotic reimplantation, as well as the multijointed angulation provided by the robotic instruments that facilitates the ureteral mobilization, submucosal tunneling, and suturing. On the other hand, the larger size of the robotic camera (12 mm) and instruments (8 or 5 mm) have the potential for increased risk of port site bladder leakage, space limitation, and inferiority in cosmesis when compared to the conventional laparoscopic camera (5 mm) and instruments (3 mm) which are usually used during conventional laparoscopic reimplantation [17]. Future development of smaller and less bulky robotic instruments may overcome these potential limitations and facilitate further adoption of the intravesical RALUR procedure.
The extravesical approach of RALUR is based on the open extravesical Lich-Gregoir procedure [4142]. Since first described by Peters [11] in 2004, the extravesical approach has been adopted at many pediatric centers for RALUR. The RALUR experience appears to be following a similar historical experience to that of the open extravesical technique, where suboptimal results were noted in the early experience [43], with eventual widespread adoption worldwide.
Even though extravesical RALUR has been utilized by many surgeons over a decade, the surgical outcomes of this procedure have been reported with variability among previously reported series (Table 2). In 2008, Casale et al. [13] reported their extravesical RALUR experience of 41 children with VUR. All of the patients had bilateral reflux, and the reflux resolution rate was 97.6%. No complications were reported except for one febrile UTI episode which occurred in the patient with persistent VUR postoperatively. No episodes of urinary retention were documented by bladder scan. In 2012, this group reported on their subsequent RALUR case series with an increased number of 150 children with bilateral VUR and at least 2 years of postoperative follow-up [18]. The reflux resolution rate was 99.3% based on postoperative VCUG. Only one patient had persistent reflux after RALUR which became downgraded from bilateral grade 5 VUR to unilateral grade 2 VUR. No patients were reported to have de novo voiding dysfunction or urinary retention when measured by objective voiding parameters and validated questionnaire. The authors concluded that bilateral nerve-sparing robotic-assisted extravesical reimplantation is associated with similar success rates as the traditional open approaches, with minimal morbidity and no voiding complications after surgery. Other subsequent series also reported similar favorable outcomes of RALUR. In 2011, Smith et al. [15] compared surgical outcomes of 25 extravesical RALUR patients with those of 25 open cross-trigonal ureteral reimplantation patients. The overall success rate was 97% for RALUR compared to 100% for open reimplantation, but with a reduced mean length of stay (33 hours vs. 53 hours) and reduced pain medication usage in the RALUR group. In 2015, Silay et al. [26] reported the outcomes of 72 children (91 ureters) who underwent extravesical RALUR. The complete resolution of VUR was observed in 97.9% with only 2 patients (2.7%) reporting development of temporary postoperative urinary retention that self-resolved within 2 weeks.
Other series reported favorable reflux resolution rates with RALUR but with uncommon but serious complications. In 2011, Marchini et al. [16] reported 20 cases of extravesical RALUR with a reflux resolution rate of 100%, but with 2 cases (10%) of ureteral injury. In 2014, Akhavan et al. [23] reported the outcomes of 78 extravesical RALUR in 50 patients. Ten patients (20%) had prior Deflux injection, and 2 (4%) had prior ureteroneocystostomy on the ipsilateral side. The reflux resolution rate was 92.3% based on postoperative VCUG, but the overall complication rate was 10% including 2 patients (4%) with ureteral obstruction and 1 (2%) with a ureteral injury, which necessitated ureteral stent placement in all 3 patients. They found no association between persistent reflux and patient gender, age, presence of voiding dysfunction, history of prior reflux surgery, bilaterality, or stent placement during surgery.
Two other series have reported suboptimal success rates with relatively high complication rates. In 2015, Grimsby et al. [25] reported on the experience at 2 institutions with extravesical RALUR. Of the 61 patients (93 ureters) who underwent RALUR, VUR resolution was noted in only 44 of 61 patients (72%), while 6 major complications (10%) were also noted including ureteral obstruction or ureteral leak. Reoperations were performed in 9 patients (11%). No specific demographic or intraoperative factor was determined to be responsible for the lower success rate. In 2016, Herz et al. [27] reported the outcomes of extravesical RALUR in 54 children with a total of 72 ureters. Overall surgical success was 85.2% of ureters and overall complication was 11% including ureteral obstruction (7.4%) or ureteral injury (3.7%). They concluded that bilateral RALUR is associated with higher failure rates, higher complication rates, higher reoperation rates, and more postoperative UTIs and nonsurgical readmissions compared with unilateral RALUR.
Several possibilities may explain the variability in the reported surgical outcomes of extravesical RALUR at different institutions. As one potential explanation, similar to all new techniques, there is most likely variability in the patient populations and in surgeon experience, where the success rates after RALUR may have been affected by case selection as well as by a surgeon's learning curve. With the adoption of RALP, the operative time of RALP significantly decreased as surgeons progressed through their learning curve, and the reduction of operative time and the potential for major complications with RALUR is expected as the learning curve is overcome [44]. Another consideration is the underestimated complexity of the extravesical RALUR procedure. Similar to the open approach, essential surgical steps for RALUR include the mobilization of the ureter proximally for approximately 4 to 5 cm to achieve a sufficient length for reimplantation which should include minimal usage of cautery and ureteral handling during distal ureteral dissection, with preservation of periureteral tissues and the delicate blood supply to the ureter. Manipulation of the ureter should also be minimized when the detrusor flaps are wrapped over the ureter during the reimplantation.
The reported complications associated with RALUR range from mild to severe [11121314151617181920212223242526272829]. The most common complication is urinary retention, which can be considered a relatively mild complication since it usually does not require an extended hospital stay or additional surgical procedures. Replacement of the Foley catheter for up to 2 weeks may be required, after which self-resolution of the retention usually occurs. After Foley catheter is removed, timed voiding and double voiding should be encouraged every 2 to 3 hours to promote bladder emptying. While urinary retention is a known risk after bilateral open ureteral reimplantation as well with an incidence as high as 26% [45], the risk may be enhanced in RALUR patients when voiding dysfunction or bladder bowel dysfunction is present. However, the reported urinary retention rate after bilateral RALUR overall appears to be lower than that for open extravesical ureteral reimplantation (estimated at approximately 10% of bilateral cases) [46]. Casale et al. suggested that the enhanced robotic visualization allowed for detection and careful manipulation of the periureteral tissue at the bladder hiatus with identification of the pelvic plexus, and therefore a decrease in the incidence of postoperative urinary retention [1318]. However, in another report, location of the pelvic plexus by recording muscle action potential of bladder smooth muscle after electric stimulation could not be identified during the assessment of bladder muscle response to electro-physiologic stimulation of the pelvic nerve plexus during RALUR [47].
Major complications after RALUR include ureteral injury with ureteral obstruction or leakage. Ureteral obstruction is commonly due to transient perioperative edema and often improves spontaneously over time. Placement of a ureteral stent can be considered when a reimplantation is performed in a solitary kidney. If ureteral obstruction persists with signs of progressive obstruction or concomitant pyelonephritis, a ureteral stent should be placed [30]. Ureteral injury can also be caused by aggressive dissection and devascularization of the ureter from excessive cauterization or an inadvertent injury to the ureter. The lack of haptic feedback during robotic surgery may contribute to the incidence of this type of injury. It may be noticed immediately during the robotic procedure, but also may have a delayed presentation with signs of abdominal distention and pain or ileus.
No consensus currently exists on which RALUR surgical technique should be used to maximize surgical outcomes, as several techniques have been associated with outcomes similar to that of open reimplantation. We previously described our modified ‘top-down’ suturing technique of interrupted sutures without the need for ureteral elevation or stent placement that is associated with low complication rates and high success rates [26]. Unlike previously described ‘bottom to top’ techniques [214849], where the detrusor muscle closure starts from the bottom to the top, our first detrusor closure stitch for the ‘top-down approach’ is placed at the superior aspect in an interrupted fashion that creates the new muscle hiatus, and which also elevates the ureter without tension or manipulation to decrease instrument-related contact with the ureter for the subsequent sutures. Gundeti et al. [29] also reported on a modified technique based on periodic review and critical analysis of video recordings of previous procedures, which increased the success rates for resolution of VUR following their technique modifications. Some of their suggestions for improving surgical outcomes include adequate lengths (4–5 cm) for the detrusor tunnel, and use of a U stitch. Some of the most important principles for operative success are based on principles commonly taught for all open reimplantation surgery such as a no-touch rule for handling the ureter, low cautery settings, and careful use of the robotic instruments that do not lead to inadvertent injury to the ureter and bladder.
Concerns have been raised as to whether RALUR is a cost-effective option when compared to open surgery due to its high capital costs and heterogeneous clinical outcomes, and several recent studies have attempted to compare the costs between RALUR and open ureteral reimplantation [325051]. However, while the estimation of actual costs can be difficult, the overall conclusions from these studies noted that the direct cost including operating room charges may be higher for RALUR, but the shorter hospital length of stay with lower hospitalization charges can result in equivalent total charges in comparison with open ureteral reimplantation. This is especially applicable in the United States where daily charges for each hospital day are in the thousands of dollars. Mahida et al. [50] compared the costs of 6 most frequently performed robotic surgeries with their similar nonrobotic procedures using patients identified in the Pediatric Health Information System database. The median hospitalization costs of RALUR and open ureteral reimplantation were $13,096 (range, $9,057–$17,890) and $8,530 (range, $6,901–$11,148) for this group, respectively [49]. It should be noted that charges/costs can vary significantly between institutions and hence may limit the applicability of this comparison. Similarly, Kurtz et al. [51] compared the cost and complications between 108 RALUR and 1,494 open ureteral reimplantation cases from a nationwide sample. The median hospital cost for RALUR was $9,128 versus $7,273 for open ureteral reimplantation, however, there was a considerable variability from the differential experience among centers expressed as complications, and the higher cost of RALUR was associated with a significantly higher rate of complications. Thus, we can expect that when the complication rate decreases with technical improvements in RALUR, the costs will also reduce to the equivalent levels for open ureteral reimplantation. In our propensity-matched study (publication pending) comparing the hospital charges for robotic (n=38) and open (n=97) ureteral reimplantations, the higher operating room charges for the RALUR were offset by the shorter hospital stays, lower hospitalization charges, and avoidance of major complications that led to equivalent overall hospital charges for the RALUR and open cohorts ($21,437 for RALUR versus $21,461 for open reimplantation).
The trifecta of outcomes for the surgical treatment of prostate cancer are cancer control, urinary continence, and preservation of erectile function. Robot-assisted laparoscopic prostatectomy has become the standard surgical option for men with prostate cancer where over 90% of surgical patients are undergoing the robotic option, since the outcomes trifecta above for radical prostatectomy can be achieved at many centers. Similar to radical prostatectomy, a proposed trifecta for surgical treatment of VUR can be equivalent success rates, equivalent hospital charges, and low complication rates similar to those of the gold standard, open ureteral reimplantation. During the past decade, RALUR has become an increasingly utilized option for the surgical management of VUR, yet the reported outcomes and complication rates have been varied among institutions. As we enter into the second decade of RALUR, it is well understood that the favorable outcomes and low complication rates for RALUR need to be comparable to open ureteral reimplantation for all surgeons and centers, and we are well on the way to achieving these goals. Educational opportunities such as robotic hands-on course and expanded training during residency/fellowship training should help to standardize RALUR techniques to those that are associated with comparable outcomes to open reimplantation. Furthermore, multi-institutional collaborations are in progress that are identifying technical factors that hopefully will lead to widespread best practices for RALUR. In addition, we expect that the further evolution of robotic technology will continued to be applied to the pediatric field that will facilitate complex procedures such as RALUR. However, the impetus to improve pediatric medical devices is difficult because of the smaller pediatric population and therefore smaller number of pediatric procedures when compared to the adult population [52]. An example is how the most recent Da Vinci robotic system (Xi) has no 5-mm instruments, while they are available for the earlier models. As pediatric specialists, we need to continue to encourage as opposed to discourage the use of new technology in children, and especially for high volume procedures such as RALUR, as only this will lead to the “next big thing” in pediatric surgical care.
From the lessons learned over the past decade, RALUR for the treatment of pediatric VUR should be reclassified as a complex reconstructive procedure in pediatric urology, as this procedure can be associated with significant complications and especially for surgeons early in their learning curve. Current efforts of identifying patient-related and intraoperative factors associated with optimal surgical outcomes provide an educational opportunity to improve the success rates associated with this procedure. Multiinstitutional collaborations are in the progress of identifying key surgical steps and technical factors with the goal of improving the surgical outcomes of RALUR for all robotic pediatric urologic surgeons including surgeons early in their learning curve. This should lead to similar outcomes and avoidance of complications for RALUR that are already associated with open ureteral reimplantation, but with the advantages of a minimally invasive procedure.
References
1. Arant BS Jr. Vesicoureteric reflux and renal injury. Am J Kidney Dis. 1991; 17:491–511. PMID: 2024650.

2. Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2016; 12. 3. DOI: 10.1001/jamaoncol.2016.5688. [Epub].
3. Subcommittee on Urinary Tract Infection. Steering Committee on Quality Improvement and Management. Roberts KB. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011; 128:595–610. PMID: 21873693.

4. Capone MA, Balestracci A, Toledo I, Martin SM. Diagnosis of vesicoureteral reflux according to the 1999 and 2011 guidelines of the Subcommittee on Urinary Tract Infection of the American Academy of Pediatrics. Arch Argent Pediatr. 2016; 114:129–134. PMID: 27079390.
5. Lewis R, Hornberger B. The current state of prostate-specific antigen testing. JAAPA. 2016; 29:51–53.

6. Sood A, Jeong W, Peabody JO, Hemal AK, Menon M. Robot-assisted radical prostatectomy: inching toward gold standard. Urol Clin North Am. 2014; 41:473–484. PMID: 25306159.
7. Baek M, Kim KD. Current surgical management of vesicoureteral reflux. Korean J Urol. 2013; 54:732–737. PMID: 24255753.

8. Elder JS, Peters CA, Arant BS Jr, Ewalt DH, Hawtrey CE, Hurwitz RS, et al. Pediatric Vesicoureteral Reflux Guidelines Panel summary report on the management of primary vesicoureteral reflux in children. J Urol. 1997; 157:1846–1851. PMID: 9112544.

9. Song SH, Kim KS. Current status of robot-assisted laparoscopic surgery in pediatric urology. Korean J Urol. 2014; 55:499–504. PMID: 25132942.

10. Cundy TP, Shetty K, Clark J, Chang TP, Sriskandarajah K, Gattas NE, et al. The first decade of robotic surgery in children. J Pediatr Surg. 2013; 48:858–865. PMID: 23583146.

11. Peters CA. Robotically assisted surgery in pediatric urology. Urol Clin North Am. 2004; 31:743–752. PMID: 15474601.

12. Peters CA, Woo R. Intravesical robotically assisted bilateral ureteral reimplantation. J Endourol. 2005; 19:618–621. PMID: 16053348.

13. Casale P, Patel RP, Kolon TF. Nerve sparing robotic extravesical ureteral reimplantation. J Urol. 2008; 179:1987–1989. PMID: 18355846.

14. Lee RS, Sethi AS, Passerotti CC, Peters CA. Robot-assisted laparoscopic nephrectomy and contralateral ureteral reimplantation in children. J Endourol. 2010; 24:123–128. PMID: 19958154.

15. Smith RP, Oliver JL, Peters CA. Pediatric robotic extravesical ureteral reimplantation: comparison with open surgery. J Urol. 2011; 185:1876–1881. PMID: 21421231.

16. Marchini GS, Hong YK, Minnillo BJ, Diamond DA, Houck CS, Meier PM, et al. Robotic assisted laparoscopic ureteral reimplantation in children: case matched comparative study with open surgical approach. J Urol. 2011; 185:1870–1875. PMID: 21421223.

17. Chan KW, Lee KH, Tam YH, Sihoe JD. Early experience in robotic-assisted laparoscopic bilateral intravesical ureteral reimplantation for vesicoureteral reflux in children. J Robot Surg. 2012; 6:259–262. PMID: 27638284.

18. Kasturi S, Sehgal SS, Christman MS, Lambert SM, Casale P. Prospective long-term analysis of nerve-sparing extravesical robotic-assisted laparoscopic ureteral reimplantation. Urology. 2012; 79:680–683. PMID: 22197530.

19. Chalmers D, Herbst K, Kim C. Robotic-assisted laparoscopic extravesical ureteral reimplantation: an initial experience. J Pediatr Urol. 2012; 8:268–271. PMID: 21641872.

20. Callewaert PR, Biallosterski BT, Rahnama'i MS, Van Kerrebroeck PE. Robotic extravesical anti-reflux operations in complex cases: technical considerations and preliminary results. Urol Int. 2012; 88:6–11. PMID: 22076472.

21. Dangle PP, Shah A, Gundeti MS. Robot-assisted laparoscopic ureteric reimplantation: extravesical technique. BJU Int. 2014; 114:630–632. PMID: 24841534.

22. Schomburg JL, Haberman K, Willihnganz-Lawson KH, Shukla AR. Robot-assisted laparoscopic ureteral reimplantation: a single surgeon comparison to open surgery. J Pediatr Urol. 2014; 10:875–879. PMID: 24766855.

23. Akhavan A, Avery D, Lendvay TS. Robot-assisted extravesical ureteral reimplantation: outcomes and conclusions from 78 ureters. J Pediatr Urol. 2014; 10:864–868. PMID: 24642080.

24. Hayashi Y, Mizuno K, Kurokawa S, Nakane A, Kamisawa H, Nishio H, et al. Extravesical robot-assisted laparoscopic ureteral reimplantation for vesicoureteral reflux: initial experience in Japan with the ureteral advancement technique. Int J Urol. 2014; 21:1016–1021. PMID: 24846118.

25. Grimsby GM, Dwyer ME, Jacobs MA, Ost MC, Schneck FX, Cannon GM, et al. Multi-institutional review of outcomes of robot-assisted laparoscopic extravesical ureteral reimplantation. J Urol. 2015; 193(5 Suppl):1791–1795. PMID: 25301094.

26. Silay MS, Baek M, Koh CJ. Robot-assisted laparoscopic extravesical ureteral reimplantation in children: top-down suturing technique without stent placement. J Endourol. 2015; 29:864–866. PMID: 25674670.

27. Herz D, Fuchs M, Todd A, McLeod D, Smith J. Robot-assisted laparoscopic extravesical ureteral reimplant: A critical look at surgical outcomes. J Pediatr Urol. 2016; 12:402.e1–402.e9. PMID: 27522319.

28. Arlen AM, Broderick KM, Travers C, Smith EA, Elmore JM, Kirsch AJ. Outcomes of complex robot-assisted extravesical ureteral reimplantation in the pediatric population. J Pediatr Urol. 2016; 12:169.e1–169.e6. PMID: 26747012.

29. Gundeti MS, Boysen WR, Shah A. Robot-assisted laparoscopic extravesical ureteral reimplantation: technique modifications contribute to optimized outcomes. Eur Urol. 2016; 70:818–823. PMID: 27036858.

30. Weiss DA, Shukla AR. The robotic-assisted ureteral reimplantation: the evolution to a new standard. Urol Clin North Am. 2015; 42:99–109. PMID: 25455176.
31. Timberlake MD, Peters CA. Current status of robotic-assisted surgery for the treatment of vesicoureteral reflux in children. Curr Opin Urol. 2017; 27:20–26. PMID: 27764016.

32. Bowen DK, Faasse MA, Liu DB, Gong EM, Lindgren BW, Johnson EK. Use of pediatric open, laparoscopic and robot-assisted laparoscopic ureteral reimplantation in the United States: 2000 to 2012. J Urol. 2016; 196:207–212. PMID: 26880414.

33. Yeung CK, Sihoe JD, Borzi PA. Endoscopic cross-trigonal ureteral reimplantation under carbon dioxide bladder insufflation: a novel technique. J Endourol. 2005; 19:295–299. PMID: 15865516.

34. Valla JS, Steyaert H, Griffin SJ, Lauron J, Fragoso AC, Arnaud P, et al. Transvesicoscopic Cohen ureteric reimplantation for vesicoureteral reflux in children: a single-centre 5-year experience. J Pediatr Urol. 2009; 5:466–471. PMID: 19428305.

35. Emir H, Mammadov E, Elicevik M, Buyukunal C, Soylet Y. Transvesicoscopic cross-trigonal ureteroneocystostomy in children: a single-center experience. J Pediatr Urol. 2012; 8:83–86. PMID: 21084225.

36. Hong CH, Kim JH, Jung HJ, Im YJ, Han SW. Single-surgeon experience with transvesicoscopic ureteral reimplantation in children with vesicoureteral reflux. Urology. 2011; 77:1465–1469. PMID: 21333340.

37. Chung MS, Han SW, Jung HJ, Im YJ, Han HH, Na JC, et al. Transvesicoscopic ureteral reimplantation in children with bilateral vesicoureteral reflux: surgical technique and results. J Laparoendosc Adv Surg Tech A. 2012; 22:295–300. PMID: 22356205.

38. Soh S, Kobori Y, Shin T, Suzuki K, Iwahata T, Sadaoka Y, et al. Transvesicoscopic ureteral reimplantation: Politano-Leadbetter versus Cohen technique. Int J Urol. 2015; 22:394–399. PMID: 25754455.

39. Liu X, Liu JH, Zhang DY, Hua Y, Lin T, Wei GH, et al. Retrospective study to determine the short-term outcomes of a modified pneumovesical Glenn-Anderson procedure for treating primary obstructing megaureter. J Pediatr Urol. 2015; 11:266.e1–266.e6. PMID: 26076822.

40. Choi H, Park JY, Bae JH. Initial experiences of laparoscopic intravesical detrusorraphy using the Politano-Leadbetter technique. J Pediatr Urol. 2016; 12:110.e1–110.e7. PMID: 26750185.

41. Gregoir W, Vanregemorter G. Congenital vesico-ureteral reflux. Urol Int. 1964; 18:122–136. PMID: 14215746.
42. Riedmiller H, Gerharz EW. Antireflux surgery: Lich-Gregoir extravesical ureteric tunnelling. BJU Int. 2008; 101:1467–1482. PMID: 18454801.

44. Lee RS, Retik AB, Borer JG, Peters CA. Pediatric robot assisted laparoscopic dismembered pyeloplasty: comparison with a cohort of open surgery. J Urol. 2006; 175:683–687. PMID: 16407025.

45. Barrieras D, Lapointe S, Reddy PP, Williot P, McLorie GA, Bägli D, et al. Urinary retention after bilateral extravesical ureteral reimplantation: does dissection distal to the ureteral orifice have a role? J Urol. 1999; 162(3 Pt 2):1197–1200. PMID: 10458465.

46. Lakshmanan Y, Fung LC. Laparoscopic extravesicular ureteral reimplantation for vesicoureteral reflux: recent technical advances. J Endourol. 2000; 14:589–593. PMID: 11030542.
47. Dangle PP, Razmaria AA, Towle VL, Frim DM, Gundeti MS. Is pelvic plexus nerve documentation feasible during robotic assisted laparoscopic ureteral reimplantation with extravesical approach? J Pediatr Urol. 2013; 9:442–447. PMID: 23218755.

49. Gundeti MS, Kojima Y, Haga N, Kiriluk K. Robotic-assisted laparoscopic reconstructive surgery in the lower urinary tract. Curr Urol Rep. 2013; 14:333–341. PMID: 23740381.

50. Mahida JB, Cooper JN, Herz D, Diefenbach KA, Deans KJ, Minneci PC, et al. Utilization and costs associated with robotic surgery in children. J Surg Res. 2015; 199:169–176. PMID: 26013442.

51. Kurtz MP, Leow JJ, Varda BK, Logvinenko T, Yu RN, Nelson CP, et al. Robotic versus open pediatric ureteral reimplantation: costs and complications from a nationwide sample. J Pediatr Urol. 2016; 12:408.e1–408.e6. PMID: 27593917.

52. Grant M, Stanasel I, Koh CJ. Pediatric medical device consortia: a novel pathway for device development for pediatric urologists and other pediatric specialists. Urol Pract. 2015; 2:206–210.
Fig. 1
Personal historical perspective of pediatric robotic surgical cases over the past decade that include intravesical RALUR (ureteral reimplantation) cases. RALUR remains a significant procedure in terms of surgical volume, and second in volume only to robotic pyeloplasty. RALUR, robot-assisted laparoscopic ureteral reimplantation.
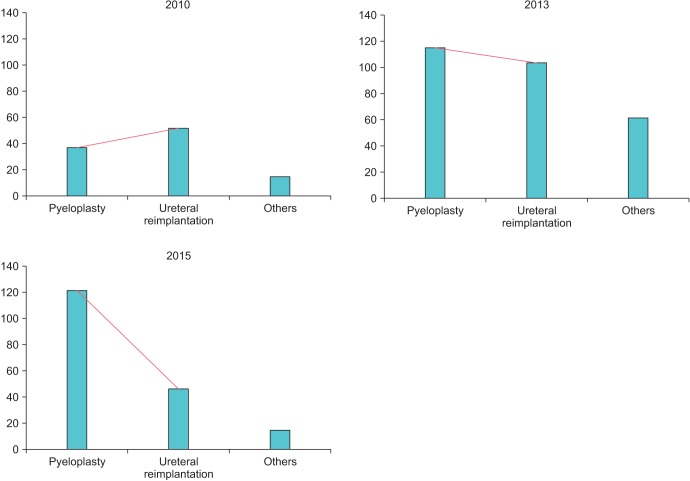
Table 1
Previously published articles on pediatric intravesical robot-assisted laparoscopic ureteral reimplantation
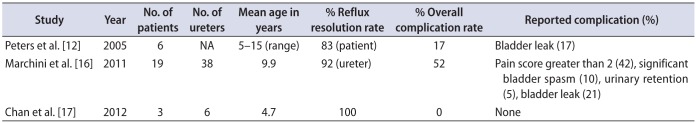
| Study | Year | No. of patients | No. of ureters | Mean age in years | % Reflux resolution rate | % Overall complication rate | Reported complication (%) |
|---|---|---|---|---|---|---|---|
| Peters et al. [12] | 2005 | 6 | NA | 5–15 (range) | 83 (patient) | 17 | Bladder leak (17) |
| Marchini et al. [16] | 2011 | 19 | 38 | 9.9 | 92 (ureter) | 52 | Pain score greater than 2 (42), significant bladder spasm (10), urinary retention (5), bladder leak (21) |
| Chan et al. [17] | 2012 | 3 | 6 | 4.7 | 100 | 0 | None |
Table 2
Previously published articles on pediatric extravesical robot-assisted laparoscopic ureteral reimplantation
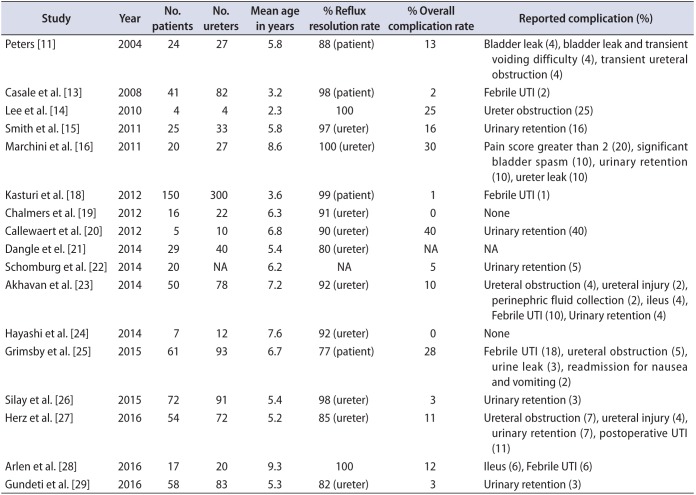
| Study | Year | No. patients | No. ureters | Mean age in years | % Reflux resolution rate | % Overall complication rate | Reported complication (%) |
|---|---|---|---|---|---|---|---|
| Peters [11] | 2004 | 24 | 27 | 5.8 | 88 (patient) | 13 | Bladder leak (4), bladder leak and transient voiding difficulty (4), transient ureteral obstruction (4) |
| Casale et al. [13] | 2008 | 41 | 82 | 3.2 | 98 (patient) | 2 | Febrile UTI (2) |
| Lee et al. [14] | 2010 | 4 | 4 | 2.3 | 100 | 25 | Ureter obstruction (25) |
| Smith et al. [15] | 2011 | 25 | 33 | 5.8 | 97 (ureter) | 16 | Urinary retention (16) |
| Marchini et al. [16] | 2011 | 20 | 27 | 8.6 | 100 (ureter) | 30 | Pain score greater than 2 (20), significant bladder spasm (10), urinary retention (10), ureter leak (10) |
| Kasturi et al. [18] | 2012 | 150 | 300 | 3.6 | 99 (patient) | 1 | Febrile UTI (1) |
| Chalmers et al. [19] | 2012 | 16 | 22 | 6.3 | 91 (ureter) | 0 | None |
| Callewaert et al. [20] | 2012 | 5 | 10 | 6.8 | 90 (ureter) | 40 | Urinary retention (40) |
| Dangle et el. [21] | 2014 | 29 | 40 | 5.4 | 80 (ureter) | NA | NA |
| Schomburg et al. [22] | 2014 | 20 | NA | 6.2 | NA | 5 | Urinary retention (5) |
| Akhavan et al. [23] | 2014 | 50 | 78 | 7.2 | 92 (ureter) | 10 | Ureteral obstruction (4), ureteral injury (2), perinephric fluid collection (2), ileus (4), Febrile UTI (10), Urinary retention (4) |
| Hayashi et al. [24] | 2014 | 7 | 12 | 7.6 | 92 (ureter) | 0 | None |
| Grimsby et al. [25] | 2015 | 61 | 93 | 6.7 | 77 (patient) | 28 | Febrile UTI (18), ureteral obstruction (5), urine leak (3), readmission for nausea and vomiting (2) |
| Silay et al. [26] | 2015 | 72 | 91 | 5.4 | 98 (ureter) | 3 | Urinary retention (3) |
| Herz et al. [27] | 2016 | 54 | 72 | 5.2 | 85 (ureter) | 11 | Ureteral obstruction (7), ureteral injury (4), urinary retention (7), postoperative UTI (11) |
| Arlen et al. [28] | 2016 | 17 | 20 | 9.3 | 100 | 12 | Ileus (6), Febrile UTI (6) |
| Gundeti et al. [29] | 2016 | 58 | 83 | 5.3 | 82 (ureter) | 3 | Urinary retention (3) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download