Abstract
Purpose
To evaluate the impact of incontinence etiology on artificial urinary sphincter (AUS) device outcomes.
Materials and Methods
We identified 925 patients who underwent primary AUS placement from 1983 to 2011. The etiology of incontinence was categorized as radical prostatectomy alone, radical prostatectomy with radiation, benign prostate resection, and those with cryotherapy as a salvage prostate cancer treatment. Hazard regression and competing risk analyses were used to determine the association of the etiology of incontinence with device outcomes.
Results
The distribution of the 4 etiologies of incontinence included: 598 patients (64.6%) treated with prostatectomy alone, 206 (22.2%) with prostatectomy and pelvic radiation therapy, 104 (11.2%) with benign prostate resection, and 17 (1.8%) with prior cryotherapy. With a median follow-up of 4.9 years (interquartile range, 1.2–8.8 years), there was significant difference in the cumulative incidence of device infection/urethral erosion events between the four etiologies (p=0.003). On multivariable analysis, prior cryotherapy (reference prostatectomy alone; hazard ratio [HR], 3.44; p=0.01), older age (HR, 1.07; p=0.0009) and history of a transient ischemic attack (HR, 2.57; p=0.04) were associated with an increased risk of device infection or erosion. Notably, pelvic radiation therapy with prostatectomy was not associated with an increased risk of device infection or erosion (reference prostatectomy alone, p=0.30).
Artificial urinary sphincter (AUS) implantation remains the preferred surgical method of treatment for males with severe stress urinary incontinence [1]. Despite its introduction over 30 years ago and widespread use, evaluation of risk factors for adverse outcomes is limited in breadth [234]. Specifically, data on the etiology of incontinence as a predictor of postoperative complications is scarce [2567].
Prior evaluations of the impact of prior pelvic radiation therapy on AUS outcomes have met with conflicting results [23568910111213]. However, these studies did not separate those with the prostate in situ, or evaluate the timing of radiotherapy (e.g., primary, adjuvant, or salvage) [235689101213]. Moreover, data evaluating outcomes in other etiologic groups such as benign prostatic resection, or cryotherapy are lacking. Recognition of these differences may be useful for risk stratification and better informing preoperative patient counseling.
Thus, in a large patient cohort with long-term follow-up, we sought to evaluate AUS outcomes among individuals who underwent radical prostatectomy (RP), RP with radiation, benign prostate resection, or cryotherapy as a salvage prostate cancer treatment.
After obtaining Institutional Review Board approval (approval number: 16-002476), we identified 1,802 male patients undergoing AUS implantation at Mayo Clinic (Rochester, MN, USA) from 1983 to 2011. Of these, 925 (51%) were primary AUS implantations. We purposely limited our study group to AUS procedures performed up to 2011 to allow for adequate patient follow-up. Patients were excluded from analysis if they underwent AUS placement secondary to neurogenic bladder, were younger than 18 years of age, were female or declined research consent.
All implanted AUS devices were American Medical Systems 800 (AMS 800; American Medical Systems, Minnetonka, MN, USA). At our institution, a perineal approach is used for all AUS placements. During the perineal dissection the bulbospongiosus muscle is preserved, followed by placement of the urethral cuff around the muscle, so as to avoid direct pressure on the corpus spongiosum tissue in an effort to prevent urethral atrophy. All patients in this series had cuffs placed in this manner. After circumferential dissection of the proximal bulbar urethra (typically between the corpora cavernosum and corpora spongiosum), the appropriate cuff is selected. In cases of severely atrophic urethral tissues (measurement less than 4.0 cm) or difficult dissection planes, we use a transcorporal approach [14].
Individual charts were reviewed to evaluate pertinent clinical and surgical comorbidities, as well repeat surgery events for erosion, infection, malfunction and urethral atrophy. The retrospective nature of this study precluded a standardized follow-up protocol in all patients. Rather, patients were evaluated 6 weeks postoperatively for device activation. All patients were then followed via office evaluation on an as needed basis as determined by continence or other device concerns. As part of our ongoing departmental AUS registry, patients are also periodically contacted by mail regarding their device function. Details regarding device survival and function were obtained from the last office examination, subsequent operative report, or written or telephone correspondence.
The objective of the study was to evaluate the impact of incontinence etiology on secondary surgery rates as a result of urethral atrophy, mechanical failure and device infection/erosion. The etiology of incontinence was categorized as RP alone, RP with pelvic radiation therapy (including as initial primary therapy, adjuvant or salvage therapy), benign prostate resection (including transurethral prostate resection or prostate photovaporization), and those who underwent cryotherapy as a salvage prostate cancer treatment. Explanation was performed for all infection/erosion events, urethral atrophy events, and instances of mechanical malfunction. Statistical analysis was performed using the SAS (SAS Institute Inc., Cary, NC, USA). Continuous features were summarized with medians and interquartile ranges (IQRs), and categorical features were summarized with frequency counts and percentages. Device survival was estimated as time from AUS implantation to subsequent repeat surgery (including explanation or device revision for any reason) using the Kaplan-Meier method. Variables were included on multivariable analysis, if there was a significant association on univariate analysis. Hazard regression and competing risk analyses were used to determine the association of the etiology of incontinence with device outcomes. All statistical tests were 2-sided, with p<0.05 considered statistically significant.
The distribution of the 4 etiologies of incontinence included: 598 patients treated with RP alone (64.6%), 206 (22.2%) with RP and pelvic radiation therapy (RP+RT), 104 (11.2%) with benign prostate resection, and 17 (1.8%) with prior prostate cryotherapy. Differences in the clinical cohorts are shown in Table 1. Notably, there were significant differences between the cohorts with regard to body mass index (BMI) (p=0.03) and coronary artery disease (p=0.001). The higher BMI median and higher frequency of CAD seem to point to salvage cryotherapy as the etiology responsible for the differences. Those with incontinence after a benign prostate resection were significantly older than the other cohorts (p=0.0001).
Overall, the median postoperative follow-up was 4.9 years (IQR, 1.2–8.8), during which time 68 patients (7.5%) experienced device infection/urethral erosions, 119 patients (13.1%) underwent revision for malfunction, and 89 (9.8%) underwent revisions for urethral atrophy. Follow-up was significantly different between the different etiology groups (p=0.007). Notably, there was no difference in overall device survival between the groups on Kaplan-Meier analysis, with 5-year survival rates of 75%, 72%, 75%, and 57% among those treated with RP only, RP+RT, benign prostatic resection, and cryotherapy, respectively (p=0.25) (Fig. 1).
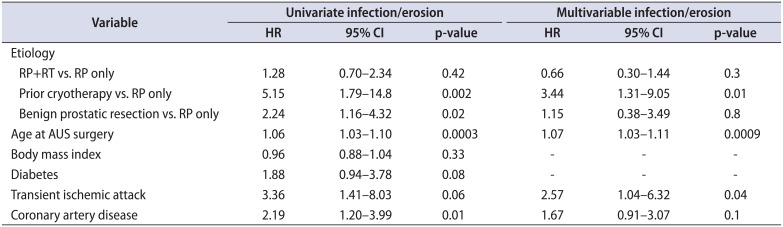
We next assessed the association of etiology of incontinence on specific device outcomes: infection/erosion, urethral atrophy, device malfunction. Notably, when evaluating specific device outcomes, a significant difference in the cumulative incidence of device infection/erosion events between the 4 etiologies was identified (p=0.003) (Fig. 2). On multivariable analysis, we found that history of cryotherapy (reference prostatectomy alone; HR, 3.44; p=0.01), older age (HR, 1.07; p=0.0009), and history of a transient ischemic attack (HR, 2.57; p=0.04) were associated with an increased risk of device infection or erosion. Notably, radiation therapy among patients that also had a prostatectomy was not associated with the risk of device infection or erosion (reference prostatectomy alone, p=0.3) (Table 2).
We found here, in a large cohort of primary AUS placements, that a history of having undergone prior cryotherapy as a salvage prostate cancer treatment was associated with a significantly increased risk of AUS device explantation for infection or urethral erosion. In contrast, a history of pelvic radiation therapy in the setting of a prostatectomy, and urinary incontinence secondary to benign prostate resection were not associated with adverse device survival. These results augment the existing literature by evaluating the outcomes of patients with etiologies of incontinence beyond prostatectomy with or without pelvic radiation therapy to benign resection and salvage therapies.
Reports regarding the etiology of incontinence, including salvage cryotherapy, on device outcomes are limited. Given the novelty of our findings with regard to salvage cryotherapy, the mechanism of the potential association has not been established. It is possible, that differences in clinical factors, such as a higher rate of coronary artery disease or higher BMI, may impact the findings. However, we attempted to account for this on multivariable analysis. An additional hypothesis may be that damage to the periurethral microcirculation as a result of cryotherapy [15] may lead to poor tissue quality and ultimately increased infection/erosion events. Further investigation on the long-term impact of salvage cryotherapy on periurethral tissues may be warranted.
To our knowledge, there is a paucity of data comparing AUS outcomes of benign versus malignant prostate interventions. While several studies exist that include these patient populations, there is no comparison of AUS outcomes amongst these groups provided [1316171819]. We were unable to find a difference in benign versus malignant prostate cancer treatment and AUS outcomes. It is worth mentioning that those with incontinence after a benign prostatic resection were significantly older than other cohorts. This may be particularly relevant when counseling patients >80 years old as our current study, as well as prior series have identified advanced age as a risk for erosion or infection compared to younger patients [20].
Notably, conflicting results regarding the impact of pelvic radiation on AUS device outcomes exist [23568910111213]. Here, we found that radiation therapy in the setting of a prior prostatectomy was not associated with an increased risk of device infection or erosion compared to those treated with prostatectomy alone. Differences in the findings of the published series may be secondary to length of follow-up available, limited comparisons accounting for patients with their prostate in situ, evaluation of the timing of radiation treatment (primary, adjuvant, salvage), or other patient and practice disparities.
Given our findings of overall device survival, we feel that a history of cryotherapy alone should not preclude a patient from AUS implantation. Rather, these patients should be appropriately counseled on the potential device survival outcomes, increased risk of infection/erosion, and should be carefully evaluated for functional status, age, and other comorbidities (particularly those with transient ischemic attack).
Limitations of our study include the fact that our patient population is a well-selected, cohort treated at a tertiary care center with a high volume AUS practice. As a result, these results cannot necessarily be extrapolated to all surgical practices. Additionally, given the retrospective nature of this study, specific details regarding prior therapies (e.g., time from treatment to AUS, radiation protocol or cryotherapy protocol) were not available. Furthermore, although we present a large cohort of patients, the subgroup of patients who underwent cryotherapy represented 1.8% of all procedures (n=17), and thus larger studies and external validation are needed. Furthermore, given the referral nature of our practice, some patient may follow up with local providers, introducing heterogeneity into patient follow up. To account for this, our departmental AUS registry sends periodic surveys to patients regarding device status.
Men who have undergone salvage cryotherapy are at significantly increased risk of AUS infection/erosion compared with those who have undergone RP alone. This information may be useful in the preoperative counseling of men undergoing primary AUS placement with a history of salvage cryotherapy.
ACKNOWLEDGMENTS
All funding was provided by the Department of Urology at Mayo Clinic in Rochester, Minnesota, USA.
References
1. Scott FB, Bradley WE, Timm GW. Treatment of urinary incontinence by an implantable prosthetic urinary sphincter. J Urol. 1974; 112:75–80. PMID: 4600662.

2. Kim SP, Sarmast Z, Daignault S, Faerber GJ, McGuire EJ, Latini JM. Long-term durability and functional outcomes among patients with artificial urinary sphincters: a 10-year retrospective review from the University of Michigan. J Urol. 2008; 179:1912–1916. PMID: 18353376.

3. Lai HH, Hsu EI, Teh BS, Butler EB, Boone TB. 13 years of experience with artificial urinary sphincter implantation at Baylor College of Medicine. J Urol. 2007; 177:1021–1025. PMID: 17296403.

4. Wang R, McGuire EJ, He C, Faerber GJ, Latini JM. Long-term outcomes after primary failures of artificial urinary sphincter implantation. Urology. 2012; 79:922–928. PMID: 22305763.

5. Bates AS, Martin RM, Terry TR. Complications following artificial urinary sphincter placement after radical prostatectomy and radiotherapy: a meta-analysis. BJU Int. 2015; 116:623–633. PMID: 25601072.

6. Brant WO, Erickson BA, Elliott SP, Powell C, Alsikafi N, Mc-Clung C, et al. Risk factors for erosion of artificial urinary sphincters: a multicenter prospective study. Urology. 2014; 84:934–938. PMID: 25109562.

7. Linder BJ, de Cogain M, Elliott DS. Long-term device outcomes of artificial urinary sphincter reimplantation following prior explantation for erosion or infection. J Urol. 2014; 191:734–738. PMID: 24018241.

8. Gomha MA, Boone TB. Artificial urinary sphincter for postprostatectomy incontinence in men who had prior radiotherapy: a risk and outcome analysis. J Urol. 2002; 167(2 Pt 1):591–596. PMID: 11792924.

9. Linder BJ, Rivera ME, Ziegelmann MJ, Elliott DS. Long-term outcomes following artificial urinary sphincter placement: an analysis of 1082 cases at Mayo Clinic. Urology. 2015; 86:602–607. PMID: 26135815.

10. Ravier E, Fassi-Fehri H, Crouzet S, Gelet A, Abid N, Martin X. Complications after artificial urinary sphincter implantation in patients with or without prior radiotherapy. BJU Int. 2015; 115:300–307. PMID: 24731208.

11. Rivera ME, Linder BJ, Ziegelmann MJ, Viers BR, Rangel LJ, Elliott DS. The impact of prior radiation therapy on artificial urinary sphincter device survival. J Urol. 2016; 195(4 Pt 1):1033–1037. PMID: 26518111.

12. Sathianathen NJ, McGuigan SM, Moon DA. Outcomes of artificial urinary sphincter implantation in the irradiated patient. BJU Int. 2014; 113:636–641. PMID: 24131859.

13. Walsh IK, Williams SG, Mahendra V, Nambirajan T, Stone AR. Artificial urinary sphincter implantation in the irradiated patient: safety, efficacy and satisfaction. BJU Int. 2002; 89:364–368. PMID: 11872025.

14. Magera JS Jr, Elliott DS. Tandem transcorporal artificial urinary sphincter cuff salvage technique: surgical description and results. J Urol. 2007; 177:1015–1019. PMID: 17296400.

15. Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998; 37:171–186. PMID: 9787063.

16. Gomes CM, Broderick GA, Sánchez-Ortiz RF, Preate D Jr, Rovner ES, Wein AJ. Artificial urinary sphincter for postprostatectomy incontinence: impact of prior collagen injection on cost and clinical outcome. J Urol. 2000; 163:87–90. PMID: 10604321.

17. Imamoglu MA, Tuygun C, Bakirtas H, Yiğitbasi O, Kiper A. The comparison of artificial urinary sphincter implantation and endourethral macroplastique injection for the treatment of postprostatectomy incontinence. Eur Urol. 2005; 47:209–213. PMID: 15661416.

18. Ramsay AK, Granitsiotis P, Conn IG. The use of the artificial urinary sphincter in the West of Scotland: a single centre 10-year experience. Scott Med J. 2007; 52:14–17.

19. Singh G, Thomas DG. Artificial urinary sphincter for postprostatectomy incontinence. Br J Urol. 1996; 77:248–251. PMID: 8800893.

20. Ziegelmann MJ, Linder BJ, Rivera ME, Viers BR, Rangel LJ, Elliott DS. Outcomes of artificial urinary sphincter placement in octogenarians. Int J Urol. 2016; 23:419–423. PMID: 26890355.

Fig. 1
Kaplan-Meier curve of overall device survival stratified by radical prostatectomy (RP), radical prostatectomy with radiation (RP+RT), benign prostate resection (TURP), and cryotherapy as a salvage prostate cancer treatment (Cryo). AUS, artificial urinary sphincter.
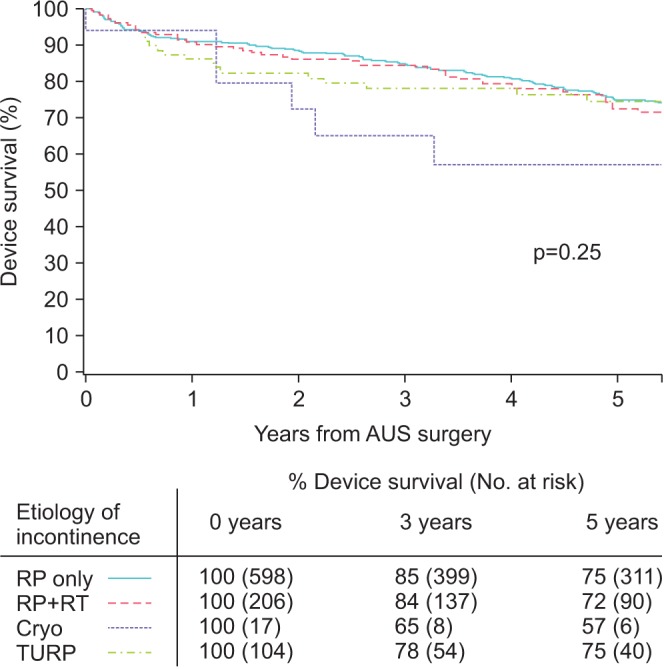
Fig. 2
Cumulative incidence curves for secondary surgery, stratified by etiology of incontinence for device infection/erosion (A), mechanical failure (B), and urethral atrophy (C). RP, radical prostatectomy; RP + RT, radical prostatectomy with radiation; TURP, benign prostate resection; Cryo, prior cryotherapy as salvage treatment.
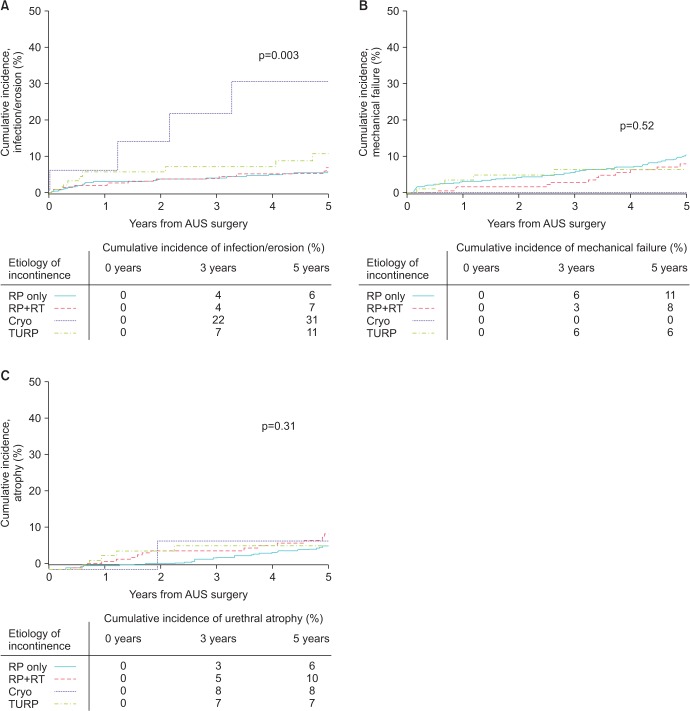
Table 1
Clinical and demographic factors of patients undergoing primary AUS placement, stratified by etiology of incontinence
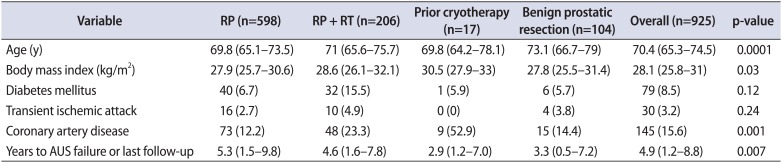
Table 2
Multivariable analysis of factors associated with device explantation for infection/erosion




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download