Abstract
Purpose
To determine the impact of lymph node density (LND) on survival after inguinal lymph node dissection (ILND) for penile cancer.
Materials and Methods
Our institutional penile cancer database was queried for patients who underwent ILND. Clinicopathologic characteristics including LND and total number of positive lymph nodes (LNs) were analyzed to determine impact on recurrence-free survival (RFS) and overall survival (OS). LND, or the percent of positive LN out of total LN, was calculated as a categorical variable at varying thresholds.
Results
Twenty-eight patients with complete follow-up were identified. Indications for ILND were stage >T2 in 20 patients (71.4%), palpable adenopathy in 7 (25%), high grade T1 in 1 (3.6%). Median node yield was 17.5 (interquartile range, 12−22), and positive LNs were found in 14 patients (50%). RFS and OS were significantly lower for patients with >15% LN density (median RFS: 62 months vs. 6.3 months, p=0.0120; median OS: 73.6 months vs. 6.3 months, p<0.001). Controlling for age, medical comorbidities, number of positive LN, T stage, pelvic LN status and indication, LN density >15% was independently associated with worse RFS (hazard ratio [HR], 3.6; p=0.04) and OS (HR, 73.6; p=0.002). The c-index for LND was higher than total positive LNs for RFS (0.64 vs. 0.54) and OS (0.79 vs. 0.61).
Penile carcinoma is an aggressive urologic malignancy in which the most significant prognostic variable is the presence and extent of lymph node involvement [1234]. Therefore, considerable interest has focused on the inguinal lymph node dissection (ILND) which provides valuable pathologic staging [567], guides adjuvant therapy [8], and offers therapeutic benefit [9]. Multiple studies, as reflected in current TNM staging for penile cancer [10], have shown that number of positive lymph node (LN) predicts recurrence-free survival (RFS) and overall survival (OS) [71112]. However, these studies do not account for the extent of ILND, masking the true extent of lymph node involvement. Recent literature has shown lymph node density (LND), defined by percentage of positive LN, is a superior prognostic tool for oncological outcomes after ILND by accounting for both extent of dissection and nodal disease burden [13141516].
While previous literature has demonstrated a prognostic benefit for LND, the studies are relatively few in number. Furthermore, there is considerable disagreement in the LND cutoff used to differentiate poor versus favorable survivability, ranging from 6.7% to 33% [131416]. The discordance may be explained by a significant variation in the total number of lymph nodes removed in previous studies, inclusion of patients with limited lymph node dissections, as well as varying statistical rationales used to calculate the cutoff. The wide range of nodes removed is also related to the inclusion of patients who simultaneously underwent pelvic lymph node dissection in patient cohorts, skewing the LND calculation.
The objective of this study was to validate the use of LND as a predictor of RFS and OS after ILND. We compare our results and analysis with the literature in an effort to reconcile the variation in cutoff LND and identify how different patient populations and statistical rationales have affected the results.
Our institutional penile cancer database was queried for patients who underwent ILND from 1988 to 2012. Demographic and pathologic characteristics were analyzed to determine impact on RFS and OS. LND or the percent of positive LN out of total LN was calculated as a categorical variable at varying thresholds of 10%, 15%, and 20%. In patients who underwent simultaneous pelvic lymph dissection, positive pelvic lymph nodes were accounted for in the multivariable model, but were not included in calculations for lymph node yield or LND. from analysis. Patients underwent bilateral modified templates in all cases. In patients with nonpalpable nodes, a superficial dissection above the fascia lata was performed. In cases with palpable adenopathy or suspicious nodes encountered during superficial dissection, a deep dissection was performed. Pelvic lymphadenectomy was performed in patients with positive deep inguinal lymph nodes or with enlarged pelvic lymph nodes on cross sectional imaging. All nodal and fibroadipose tissue was completely embedded prior to pathologist analysis. At the time of primary tumor and lymph node dissection, specimens were reviewed both by general surgical pathologists and at a daily consensus surgical pathology conference attended by at least one senior urological pathologist for issues relating to grade, stage and margins.
Descriptive statistics were used to summarize patient characteristics and pathologic features. Continuous variables were compared with the Wilcoxon rank-sum or Kruskal-Wallis tests and categorical variables with the chi-square test.
Survival analysis was performed using the Kaplan-Meier method to determine RFS. The log rank test was used to compare survival curves. OS was calculated from the date of surgery to death from any cause or last follow-up. RFS was calculated from the date of surgery to local or distant recurrence or death from cancer. All patients were prescribed a follow-up regimen based on the National Comprehensive Cancer Network guidelines with physical exam every 3–6 months, depending on nodal stage. Cancer and vital status were determined by both clinical follow-up at Johns Hopkins University and by a query of the Social Security Death Index. Multivariable Cox proportional hazard models including primary tumor stage, patient age, medical comorbidities, pelvic lymph node status and total number of positive LN were constructed to adjust for potential confounding. Finally, Harrell's c index for OS and RFS were calculated for both LND and total number of positive nodes to determine which variable is the superior predictor of RFS and OS. All statistical analysis was performed with Stata 13 (StataCorp., College Station, TX, USA). Two-sided p values <0.05 were considered significant.
Twenty-eight patients with complete follow-up were identified. Mean follow-up was 34 months (range, 1–174 months). Table 1 lists the clinicopathologic characteristics of the cohort. Indications for ILND were stage >pT2 in 20 patients (71.4%), palpable adenopathy in 7 (25%), and high grade T1 in 1 (3.6%). Of the patients with palpable lymph nodes, none had fixed nodes on physical exam. No patients underwent neoadjuvant chemotherapy or radiation therapy preoperatively. Two patients underwent salvage chemotherapy after recurrence, and 6 patients underwent pelvic lymph node dissection for clinically enlarged pelvic lymph nodes.
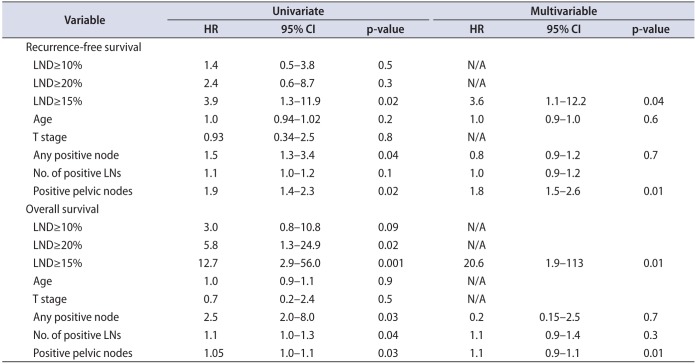
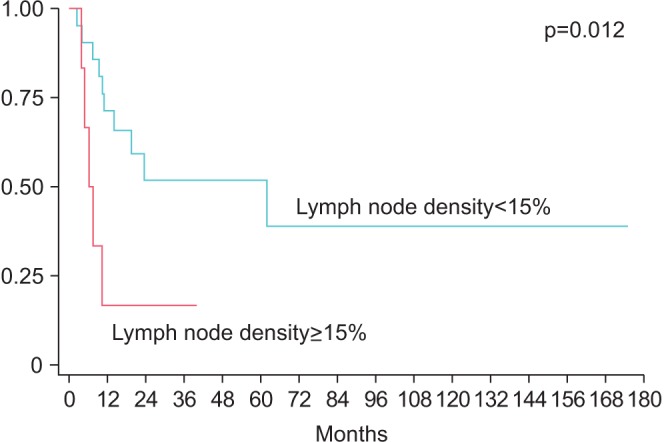
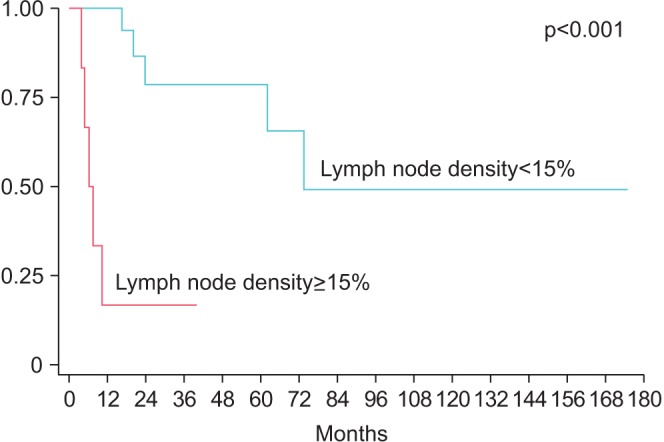
Median node yield after ILND was 17.5 (IQR, 12−22), and positive lymph nodes were found in 14 patients (50%). LND was >10% in 10 patients, >15% in six patients, >20% in 4 patients. LND thresholds of 10% and 20% were not predictive of RFS (p=0.57 and p=0.15, respectively), however RFS was significantly lower for patients with >15% LND (median survival: 62 months vs. 6.3 months, p=0.0120) (Fig. 1). Actuarial 5-year RFS was 51.9% for patients with LND<15% vs. 0% for patients with LND>15%. Similarly, OS was lower in patients with LND>15% (median OS: 73.6 months vs. 6.3 months, p<0.001) (Fig. 2). Actuarial 5-year OS was 78.7% for patients with LND<15% vs. 0% for patients with LND>15%. In a univariate Cox proportional hazard model, LND>15% was predictive of RFS (hazard ratio [HR], 3.9; 95% confidence interval [CI], 1.3–11.9, p=0.019) and OS (HR, 12.7; 95% CI, 2.9–56.0; p=0.001). Absolute number of positive lymph nodes was predictive of OS (HR, 1.14; 95% CI, 1.01–1.30; p=0.037), but not predictive of RFS (HR, 1.1; 95% CI, 0.96–1.23; p=0.18) or OS. Total LNs removed did not correlate with either RFS or OS. Controlling for age, medical comorbidities, number of positive LN, positive pelvic LN, T stage and indication, LND>15% was independently associated with worse RFS (HR, 3.6; 95% CI, 1.1–12.2; p=0.04) and OS (HR, 73.6; 95% CI, 4.8–113; p=0.002) (Table 2). Absolute number of positive lymph nodes was not predictive of OS or RFS in the multivariate model. Harrel c index for RFS was 0.64 for LND compared to 0.54 for total positive LN, indicating a 10% higher concordance for LND and RFS, than total positive LN and RFS. Likewise, the c index for OS was 0.79 for LND compared to 0.61 for for total positive LN, indicating an 18% higher concordance.
The presence and extent of disease in the inguinal lymph nodes is the most prognostic factor for survival in penile cancer [123]. In the current study, we validate the observation that LND is a predictor of worse disease. However, initial studies have shown LND cutoffs varied widely in the cutoff used to differentiate worse prognosis [13141516]. Our results identified that a cutoff LND>15% was associated with poorer prognosis. Our data provide further validation of LND's use as a prognostic tool for penile cancer and corroborates a relevant cutoff for clinical use. More significantly, we found that LND outperformed total number of positive lymph nodes both in multivariate Cox proportional hazard model and using Harrel C index for both RFS and OS.
LND's value exists in the combination of both nodal disease burden and extent of LND in a single variable. Nodal burden, represented as number of positive LN, has been shown to be associated with poorer prognosis [71112]. Pandey et al. [2] identified a 75.6%, 8.4%, and 0% 5-year survival with patients who had 1–3, 4–5, and >5 nodes, respectively. However, in this study we did not find an association with total positive nodes removed and RFS or OS. Similar to other solid tumors [17181920], LND has been shown to be a better prognostic tool than LN number as well as the current TNM staging [15] for penile cancer which can account for extent of ILND. The limited studies investigating LND have identified a survival cutoff, which has ranged widely from 6.7%–33% to differentiate favorable versus poor prognosis [13141516]. Our results fall within the wide range of cutoff values and show RFS was significantly worse with LND>15%.
Our results are very similar to Li et al. [13] and Lughezzani et al. [14] who identified LND cutoffs of 16% and 22%, respectively, to delineate poor versus favorable prognosis. Li et al. [13], in a cohort of 71 pN+ patients, showed the 16% LND cutoff separated a 5-year disease-specific survival (DSS) of 81.2% and 24.4% which in multivariate analysis was also independently associated with worse DSS with a HR of 4.31. These outcomes are similar to our 5-year RFS outcomes of 51.9% for patients with LND<15% vs 0% for patients with LND>15%. Similarly, Lughezzani et al. [14] identified on multivariate analysis that patients with LND≥22% had a 4.55-fold worse CSS. These studies matched our HR of 5.5 for RSS in our multivariate analysis when using a LND>15%. Interestingly, a higher LN threshold of 20% was not associated with RFS in our study, but this was likely due to the small number of patients with LND>20%.
Zhu et al. [16], using patients from the Surveillance, Epidemiology and End Results database, calculated a LND cutoff of 33%, however a significant proportion of their patients had insufficient lymph node dissections. While their median lymph nodes removed (16 LN) was nearly identical to ours (17.5 LN), the IQR of 5 to 27.7 LNs highlighted the inadequacy of many lymph node dissections which would both falsely elevate LND calculations as well as skew survival statistics. Svatek et al. [15] similarly affected its LND denominator with the inclusion of LNs received from pelvic lymph node dissections in 33% of patients, which explains the exceedingly high 32 median nodes removed (IQR, 21–38). Furthermore, the authors stratified survival outcomes simply based on its median LND of 6.7%, which limits its clinical applicability.
In our study, as well as the majority of papers, LND cutoff was calculated based on the identification of the LND value of maximal significance. While this method of choosing a cutoff has clinical utility for both clinicians and patients for survival prognostication, it may not be well suited for guiding practical decision-making. A more suitable LND cutoff would be aimed at the identification of a threshold value associated with low risk disease, represented by a very low recurrence rate. Lughezzani et al. [14] attempted to ascertain a more clinically relevant LND cutoff by using a 20% 5-year recurrence probability as a target for low risk disease. However the 5% LND cutoff used did not reach statistical significance, highlighting the size limitation of many penile cancer patient cohorts. Larger, prospective studies are required to calculate a clinically useful LND cutoff prior to its use in treatment algorithms to guide more extensive lymph node dissection, adjuvant therapy, as well as surveillance protocols.
There are some technical challenges to use LND that are worth noting. In patients with bulky adenopathy, nodes can be matted and an accurate node number cannot be determined. Our study population did not include any patients with matted nodes that could skew our calculations. Another potential challenge is the manner in which the tissue is processed. If there is an attempt to isolate lymph nodes from fibroadipose tissue, small positive could be overlooked. Our institutional practice is to completely embed all tissue which we believe allows a more thorough analysis.
Our study was limited by the retrospective nature as well as the small size of our patient cohort and may limit the prognostic significance of our results. Additionally, during the study period, it was the practice at our institution to proceed to ILND in patients with high-risk features or palpable adenopathy, thus the use of neoadjuvant chemotherapy may influence results of LND in other cohorts who use this multimodal approach. Our previous practice patterns were to use salvage chemotherapy at the first sign of new local recurrence or distant metastatic disease, thus more aggressive utilization of adjuvant chemotherapy may also influence the results of this study. Since 2012, we have increased our utilization of adjuvant chemotherapy in men with lymph node positive disease. The evolving management of penile cancer may change the prognostic significance of our LND cutoff, and additional studies are needed to validate this finding in other cohorts.
In our small, retrospective cohort, we identified a LND> 15% as being associated with worse RFS, providing further evidence of LND's use as a predictive tool for penile cancer. Multi-institutional studies are needed to identify clinically relevant prognostic data for this uncommon disease.
ACKNOWLEDGMENTS
This study was funded in part by a grant from the National Cancer Institute (P30CA006973).
References
1. Leijte JA, Gallee M, Antonini N, Horenblas S. Evaluation of current TNM classification of penile carcinoma. J Urol. 2008; 180:933–938. PMID: 18635216.

2. Pandey D, Mahajan V, Kannan RR. Prognostic factors in nodepositive carcinoma of the penis. J Surg Oncol. 2006; 93:133–138. PMID: 16425300.

3. Protzel C, Alcaraz A, Horenblas S, Pizzocaro G, Zlotta A, Hakenberg OW. Lymphadenectomy in the surgical management of penile cancer. Eur Urol. 2009; 55:1075–1088. PMID: 19264390.

4. Ornellas AA, Kinchin EW, Nóbrega BL, Wisnescky A, Koifman N, Quirino R. Surgical treatment of invasive squamous cell carcinoma of the penis: Brazilian National Cancer Institute long-term experience. J Surg Oncol. 2008; 97:487–495. PMID: 18425779.

5. Ornellas AA, Seixas AL, de Moraes JR. Analyses of 200 lymphadenectomies in patients with penile carcinoma. J Urol. 1991; 146:330–332. PMID: 1830347.

6. Ravi R. Correlation between the extent of nodal involvement and survival following groin dissection for carcinoma of the penis. Br J Urol. 1993; 72(5 Pt 2):817–819. PMID: 8281416.

7. Zhu Y, Ye DW, Yao XD, Zhang SL, Dai B, Zhang HL. New N staging system of penile cancer provides a better reflection of prognosis. J Urol. 2011; 186:518–523. PMID: 21679992.

8. Hakenberg OW, Compérat EM, Minhas S, Necchi A, Protzel C, Watkin N, et al. EAU guidelines on penile cancer: 2014 update. Eur Urol. 2015; 67:142–150. PMID: 25457021.

9. Johnson TV, Hsiao W, Delman KA, Jani AB, Brawley OW, Master VA. Extensive inguinal lymphadenectomy improves overall 5-year survival in penile cancer patients: results from the Surveillance, Epidemiology, and End Results program. Cancer. 2010; 116:2960–2966. PMID: 20564401.
10. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–1474. PMID: 20180029.

11. Liu JY, Li YH, Zhang ZL, Yao K, Ye YL, Xie D, et al. The risk factors for the presence of pelvic lymph node metastasis in penile squamous cell carcinoma patients with inguinal lymph node dissection. World J Urol. 2013; 31:1519–1524. PMID: 23455885.

12. Zhu Y, Gu CY, Ye DW. Population-based assessment of the number of lymph nodes removed in the treatment of penile squamous cell carcinoma. Urol Int. 2014; 92:186–193. PMID: 24246932.

13. Li ZS, Yao K, Chen P, Zou ZJ, Qin ZK, Liu ZW, et al. Disease-specific survival after radical lymphadenectomy for penile cancer: prediction by lymph node count and density. Urol Oncol. 2014; 32:893–900. PMID: 24994488.
14. Lughezzani G, Catanzaro M, Torelli T, Piva L, Biasoni D, Stagni S, et al. Relationship between lymph node ratio and cancer-specific survival in a contemporary series of patients with penile cancer and lymph node metastases. BJU Int. 2015; 116:727–733. PMID: 24128128.

15. Svatek RS, Munsell M, Kincaid JM, Hegarty P, Slaton JW, Busby JE, et al. Association between lymph node density and disease specific survival in patients with penile cancer. J Urol. 2009; 182:2721–2727. PMID: 19837433.

16. Zhu Y, Gu CY, Ye DW. Validation of the prognostic value of lymph node ratio in patients with penile squamous cell carcinoma: a population-based study. Int Urol Nephrol. 2013; 45:1263–1271. PMID: 23877663.

17. Daneshmand S, Quek ML, Stein JP, Lieskovsky G, Cai J, Pinski J, et al. Prognosis of patients with lymph node positive prostate cancer following radical prostatectomy: long-term results. J Urol. 2004; 172(6 Pt 1):2252–2255. PMID: 15538242.

18. Kassouf W, Leibovici D, Munsell MF, Dinney CP, Grossman HB, Kamat AM. Evaluation of the relevance of lymph node density in a contemporary series of patients undergoing radical cystectomy. J Urol. 2006; 176:53–57. PMID: 16753366.

19. Palapattu GS, Allaf ME, Trock BJ, Epstein JI, Walsh PC. Prostate specific antigen progression in men with lymph node metastases following radical prostatectomy: results of long-term followup. J Urol. 2004; 172(5 Pt 1):1860–1864. PMID: 15540739.

20. Stein JP, Cai J, Groshen S, Skinner DG. Risk factors for patients with pelvic lymph node metastases following radical cystectomy with en bloc pelvic lymphadenectomy: concept of lymph node density. J Urol. 2003; 170:35–41. PMID: 12796639.
Table 1
Clinicopathologic characteristics of 28 men undergoing ILND stratified by LND>15%
Table 2
Cox proportional hazard models for overall and recurrence-free survival using a lymph node density threshold of 15%
In patients with penile cancer, the status of lymph node (LN) metastases based on the TNM staging system is one of the most critical prognosticators of oncological outcomes [1]. Recently, lymph node density (LND) has been advocated as a promising predictor for survival outcomes after surgery in patients with penile cancer since first being reported by the MD Anderson Cancer Center group, similar to other malignancies such as bladder cancer [2,3].
LND is defined as the ratio of positive LNs to the total removed LNs, which is used as an indicator of disease burden by quantifying nodal involvement by tumors and therefore the actual extent of lymphadenectomy required [4]. Li et al. [5] recently reported that an LND above a 16% cutoff value was significantly associated with a better disease-specific survival (DSS) rate in 146 patients with penile cancer, and it was finally identified as an independent prognosticator of DSS by multivariate analysis (hazard ratio [HR], 4.31; p=0.009). Lughezzani et al. [6] also showed that LND (cutoff point=22%) proved to be a better predictor for DSS (HR, 4.58; p<0.001) than the 7th TNM nodal staging system (HR, 2.57; p=0.161), by evaluating 81 patients with penile cancer. In this context, the European Association of Urology released new guidelines for penile cancer in 2014, stating its prognostic impact [1]. Conversely, recent data from 182 patients with penile cancer undergoing LN dissection exhibited that a clinical N3 tumor, high number of positive LNs (≥3), and the presence of extranodal extension, but not LND (≥5.2%), remained adverse factors associated with local recurrence by multivariate analysis [7]. Despite the accumulating evidence on the prognostic value of LND, the optimal cutoff value of LND for predicting the survival in the patients with penile cancer is without consensus.
To answer this ill-defined issue, Ball et al. [8] analyzed 28 patients with penile cancer who underwent inguinal LN dissection from 1988 to 2012, and reported that an LND of ≥15% was identified as an independent predictor of recurrence-free survival and overall survival by multivariate analysis. Although this study was limited by the small sample size and unavoidable selection biases owing to its retrospective nature, it is noteworthy that the authors suggested the optimal cutoff point for LND, and that its prognostic significance was confirmed by multivariate analysis and Harrell's c-index.
In summary, the present study by Ball et al. [8] further adds validated results to the previous evidences that LND is a good indicator of the survival outcomes of patients with penile cancer. Thus, I believe that clinicians should now consider the use of LND along with the contemporary TNM staging system, as a powerful predictive factor for patients with penile cancer in real-world practice.
References
1. Hakenberg OW, Compérat EM, Minhas S, Necchi A, Protzel C, Watkin N. . EAU guidelines on penile cancer: 2014 update. Eur Urol. 2015; 67:142–150. PMID: 25457021.

2. Ku JH, Kang M, Kim HS, Jeong CW, Kwak C, Kim HH. Lymph node density as a prognostic variable in node-positive bladder cancer: a meta-analysis. BMC Cancer. 2015; 15:447. PMID: 26027955.

3. Svatek RS, Munsell M, Kincaid JM, Hegarty P, Slaton JW, Busby JE, et al. Association between lymph node density and disease specific survival in patients with penile cancer. J Urol. 2009; 182:2721–2727. PMID: 19837433.

4. Stein JP, Cai J, Groshen S, Skinner DG. Risk factors for patients with pelvic lymph node metastases following radical cystectomy with en bloc pelvic lymphadenectomy: concept of lymph node density. J Urol. 2003; 170:35–41. PMID: 12796639.
5. Li ZS, Yao K, Chen P, Zou ZJ, Qin ZK, Liu ZW, et al. Disease-specific survival after radical lymphadenectomy for penile cancer: prediction by lymph node count and density. Urol Oncol. 2014; 32:893–900. PMID: 24994488.
6. Lughezzani G, Catanzaro M, Torelli T, Piva L, Biasoni D, Stagni S, et al. Relationship between lymph node ratio and cancer-specific survival in a contemporary series of patients with penile cancer and lymph node metastases. BJU Int. 2015; 116:727–733. PMID: 24128128.

7. Reddy JP, Pettaway CA, Levy LB, Pagliaro LC, Tamboli P, Rao P, et al. Factors associated with regional recurrence after lymph node dissection for penile squamous cell carcinoma. BJU Int. 2016; 10. 18. [Epub]. DOI: 10.1111/bju.13686.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download