1. Tang Y, Li J, Pu C, Bai Y, Yuan H, Wei Q, et al. Bipolar transurethral resection versus monopolar transurethral resection for benign prostatic hypertrophy: a systematic review and meta-analysis. J Endourol. 2014; 28:1107–1114. PMID:
24754254.

2. Mamoulakis C, Ubbink DT, de la Rosette JJ. Bipolar versus monopolar transurethral resection of the prostate: a systematic review and meta-analysis of randomized controlled trials. Eur Urol. 2009; 56:798–809. PMID:
19595501.

3. Rassweiler J, Teber D, Kuntz R, Hofmann R. Complications of transurethral resection of the prostate (TURP)--incidence, management, and prevention. Eur Urol. 2006; 50:969–979. PMID:
16469429.

4. Kaplan SA. Transurethral resection of the prostate--is our gold standard still a precious commodity? J Urol. 2008; 180:15–16. PMID:
18485411.

5. Reich O, Gratzke C, Stief CG. Techniques and long-term results of surgical procedures for BPH. Eur Urol. 2006; 49:970–978. PMID:
16481092.

6. Issa MM. Technological advances in transurethral resection of the prostate: bipolar versus monopolar TURP. J Endourol. 2008; 22:1587–1595. PMID:
18721041.

7. Issa MM, Young MR, Bullock AR, Bouet R, Petros JA. Dilutional hyponatremia of TURP syndrome: a historical event in the 21st century. Urology. 2004; 64:298–301. PMID:
15302482.

8. Tefekli A, Muslumanoglu AY, Baykal M, Binbay M, Tas A, Altunrende F. A hybrid technique using bipolar energy in transurethral prostate surgery: a prospective, randomized comparison. J Urol. 2005; 174(4 Pt 1):1339–1343. PMID:
16145415.

9. Autorino R, Damiano R, Di Lorenzo G, Quarto G, Perdonà S, D'Armiento M, et al. Four-year outcome of a prospective randomised trial comparing bipolar plasmakinetic and monopolar transurethral resection of the prostate. Eur Urol. 2009; 55:922–929. PMID:
19185975.

10. Xie CY, Zhu GB, Wang XH, Liu XB. Five-year follow-up results of a randomized controlled trial comparing bipolar plasmakinetic and monopolar transurethral resection of the prostate. Yonsei Med J. 2012; 53:734–741. PMID:
22665339.

11. Mamoulakis C, Schulze M, Skolarikos A, Alivizatos G, Scarpa RM, Rassweiler JJ, et al. Midterm results from an international multicentre randomised controlled trial comparing bipolar with monopolar transurethral resection of the prostate. Eur Urol. 2013; 63:667–676. PMID:
23102675.

12. Komura K, Inamoto T, Takai T, Uchimoto T, Saito K, Tanda N, et al. Incidence of urethral stricture after bipolar transurethral resection of the prostate using TURis: results from a randomised trial. BJU Int. 2015; 115:644–652. PMID:
24909399.

13. de Sio M, Autorino R, Quarto G, Damiano R, Perdonà S, di Lorenzo G, et al. Gyrus bipolar versus standard monopolar transurethral resection of the prostate: a randomized prospective trial. Urology. 2006; 67:69–72. PMID:
16413335.

14. Huang X, Wang L, Wang XH, Shi HB, Zhang XJ, Yu ZY. Bipolar transurethral resection of the prostate causes deeper coagulation depth and less bleeding than monopolar transurethral prostatectomy. Urology. 2012; 80:1116–1120. PMID:
22990062.

15. Huang X, Wang XH, Wang HP, Qu LJ. Comparison of the microvessel diameter of hyperplastic prostate and the coagulation depth achieved with mono- and bipolar transurethral resection of the prostate. A pilot study on hemostatic capability. Scand J Urol Nephrol. 2008; 42:265–268. PMID:
17934987.

16. Mamoulakis C, Trompetter M, de la Rosette J. Bipolar transurethral resection of the prostate: the ‘golden standard’ reclaims its leading position. Curr Opin Urol. 2009; 19:26–32. PMID:
19057207.

17. Bhansali M, Patankar S, Dobhada S, Khaladkar S. Management of large (>60 g) prostate gland: PlasmaKinetic Superpulse (bipolar) versus conventional (monopolar) transurethral resection of the prostate. J Endourol. 2009; 23:141–145. PMID:
19178175.
18. Michielsen DP, Debacker T, De Boe V, Van Lersberghe C, Kaufman L, Braeckman JG, et al. Bipolar transurethral resection in saline--an alternative surgical treatment for bladder outlet obstruction? J Urol. 2007; 178:2035–2039. PMID:
17869297.

19. Michielsen DP, Coomans D. Urethral strictures and bipolar transurethral resection in saline of the prostate: fact or fiction? J Endourol. 2010; 24:1333–1337. PMID:
20583960.

20. Chen Q, Zhang L, Fan QL, Zhou J, Peng YB, Wang Z. Bipolar transurethral resection in saline vs traditional monopolar resection of the prostate: results of a randomized trial with a 2-year follow-up. BJU Int. 2010; 106:1339–1343. PMID:
20477825.

21. Erturhan S, Erbagci A, Seckiner I, Yagci F, Ustun A. Plasmakinetic resection of the prostate versus standard transurethral resection of the prostate: a prospective randomized trial with 1-year follow-up. Prostate Cancer Prostatic Dis. 2007; 10:97–100. PMID:
16926854.

22. Hu Y, Dong X, Wang G, Huang J, Liu M, Peng B. Five-year follow-up study of transurethral plasmakinetic resection of the prostate for benign prostatic hyperplasia. J Endourol. 2016; 30:97–101. PMID:
26352136.

23. Wendt-Nordahl G, Häcker A, Reich O, Djavan B, Alken P, Michel MS. The Vista system: a new bipolar resection device for endourological procedures: comparison with conventional resectoscope. Eur Urol. 2004; 46:586–590. PMID:
15474267.

24. Faul P, Schlenker B, Gratzke C, Stief CG, Reich O, Hahn RG. Clinical and technical aspects of bipolar transurethral prostate resection. Scand J Urol Nephrol. 2008; 42:318–323. PMID:
18622807.

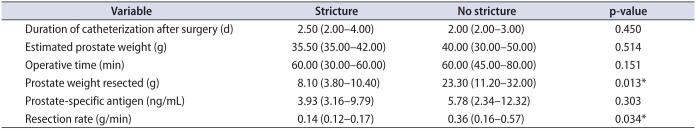
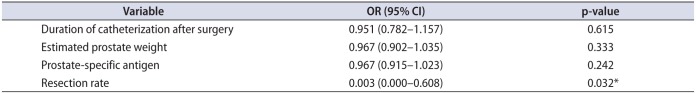
25. Tao H, Jiang YY, Jun Q, Ding X, Jian DL, Jie D, et al. Analysis of risk factors leading to postoperative urethral stricture and bladder neck contracture following transurethral resection of prostate. Int Braz J Urol. 2016; 42:302–311. PMID:
27256185.