This article has been corrected. See "CORRIGENDUM: Correction of the Figure. Robot-assisted radical prostatectomy has lower biochemical recurrence than laparoscopic radical prostatectomy: Systematic review and meta-analysis" in Volume 58 on page 383.
Abstract
Purpose
To assess the effectiveness and safety of robot-assisted radical prostatectomy (RARP) versus laparoscopic radical prostatectomy (LRP) in the treatment of prostate cancer.
Materials and Methods
Existing systematic reviews were updated to investigate the effectiveness and safety of RARP. Electronic databases, including Ovid MEDLINE, Ovid Embase, the Cochrane Library, KoreaMed, Kmbase, and others, were searched through July 2014. The quality of the selected systematic reviews was assessed by using the revised assessment of multiple systematic reviews (R-Amstar) and the Cochrane Risk of Bias tool. Meta-analysis was performed by using Revman 5.2 (Cochrane Community) and Comprehensive Meta-Analysis 2.0 (CMA; Biostat). Cochrane Q and I2 statistics were used to assess heterogeneity.
Results
Two systematic reviews and 16 additional studies were selected from a search performed of existing systematic reviews. These included 2 randomized controlled clinical trials and 28 nonrandomized comparative studies. The risk of complications, such as injury to organs by the Clavien-Dindo classification, was lower with RARP than with LRP (relative risk [RR], 0.44; 95% confidence interval [CI], 1.23–0.85; p=0.01). The risk of urinary incontinence was lower (RR, 0.43; 95% CI, 0.31–0.60; p<0.000001) and the potency rate was significantly higher with RARP than with LRP (RR, 1.38; 95% CI, 1.11–1.70; I2=78%; p=0.003). Regarding positive surgical margins, no significant difference in risk between the 2 groups was observed; however, the biochemical recurrence rate was lower after RARP than after LRP (RR, 0.59; 95% CI, 0.48–0.73; I2=21%; p<0.00001).
Radical prostatectomy has historically been the preferred treatment option for patients with localized prostate cancer. However, surgical innovations to reduce blood loss and hasten the recovery rate have led to the introduction of laparoscopic radical prostatectomy (LRP) followed by robot-assisted radical prostatectomy (RARP) as alternatives to open surgery [12]. RARP was introduced to decrease the difficulty in performing complex laparoscopic procedures such as urethral anastomosis. The robotic platform provided several advantages over LRP, such as seven degrees of freedom, tremor filtration, a three-dimensional magnified view, and preferred ergonomics [3]. Therefore, robot-assisted surgery has become popular in Korea, the United States, and Europe [14]. However, this trend has occurred despite a lack of high-quality evidence supporting improvement in outcomes.
Randomized controlled trials comparing the safety and effectiveness of RARP and LRP are limited. Therefore, high-level evidence is a requisite for clinicians needing recent evidence on the treatment of prostate cancer. The primary objective of this study was to determine whether RARP is more effective than LRP in the treatment of prostate cancer in terms of functional, oncological, and perioperative outcomes.
Eligible studies included randomized controlled trials and prospective and retrospective cohort studies comparing RARP and LRP. A study was excluded if it did not report any outcomes of interest or functional and oncological outcomes.
We searched electronic databases for reviews published through July 2014, including Ovid MEDLINE (Ovid, New York, NY, USA), Ovid EMBASE (Ovid), the Cochrane Library (London, United Kingdom), KoreaMed (KAMJE, Seoul, Korea), Kmbase (MedRIC, Chungbuk, Korea), KISS (Korean Studies Information Co, Paju, Korea), RISS (KERIS, Daegu, Korea), and KisTi (KISTI, Daejeon, Korea). Patient-related search terms (prostatic neoplasm, prostatic cancer, prostatic carcinoma, prostatic tumor), and intervention-related search terms (robotics, computer-assisted surgery, telerobot, remote operation, remote surgery, da Vinci) were combined.
Two independent reviewers selected the studies, extracted data, and performed quality assessments. The authors assessed the relevance and quality of the selected systematic reviews related to the research question through the revised assessment of multiple systematic reviews (R-Amstar). For the prospective randomized controlled clinical studies, the Cochrane Collaboration's tool for assessing risk of bias was used to perform the quality evaluations. For the nonrandomized studies on the final selected literature, a revised risk of bias was used to perform the quality evaluations. Functional and oncologic outcomes, as well as postoperative complications and perioperative results (operation duration, length of stay), were calculated and compared between the groups.
Meta-analysis was conducted by using RevMan 5.2 (Cochrane Community, London, United Kingdom) and Comprehensive Meta-Analysis 2.0 (CMA; Biostat, Englewood, NJ, USA). The Cochrane Q and I2 statistics were used to assess statistical heterogeneity. The results were expressed as weighted means and standardized mean differences for continuous outcomes and as relative risk (RR) and 95% confidence intervals (CIs) for dichotomous variables. For dichotomous variables, the random effect model of Mantel-Haenszel was used, and for continuous data, the random effect model of the inverse-variance method was used. Publication bias was tested by using a funnel plot and Egger's test. The statistical analyses were reviewed by a statistician with previous meta-analysis experience.
For the RCTs, there was a low risk of bias in sequence generation, blinding, selective report, and other biases. However, the allocation of concealment was uncertain, and incomplete outcome data were at a high risk of bias. In the cohort studies, sequence generation and allocation of concealment, which are important factors in the quality assessment of therapeutic publications, were at a high risk of bias.
The risk of complications, such as bladder neck contracture (RR, 0.40; 95% CI, 0.17–0.92; p=0.03), organ injury (RR, 0.23; 95% CI, 0.11–0.49; p=0.0002), and other major complications (Clavien-Dindo III–V) according to the Clavien-Dindo classification (RR, 0.44; 95% CI, 0.23–0.85; p=0.01), was lower for RARP than for LRP. Anastomosis site leakage (RR, 0.67; 95% CI, 0.42–1.08; p=0.10) and the rates of infection (RR, 1.21; 95% CI, 0.84–1.76; p=0.31), ileus (RR, 0.73; 95% CI, 0.38–1.40; p=0.34), and pulmonary embolism (RR, 1.20; 95% CI, 0.22–6.51; p=0.83) were not significantly different between the groups (Fig. 2). No significant difference in the conversion rate (RR, 0.70; 95% CI, 0.25–1.95; p=0.50) was observed. RARP carried a lower risk of transfusion than LRP (RR, 0.70; 95% CI, 0.54–0.91; I2=44%; p=0.007).
The operation time for RARP was shorter than that for LRP (RR, −18.74; 95% CI, −32.15 to −5.33; p=0.006), but the statistical heterogeneity was high (χ2=527.29, df=22, p<0.00001, I2=96%). The hospital stay following RARP was 1.53 days shorter than that following LRP. The results of the subgroup analysis according to region showed a mean difference of −1.13 (95% CI, 2.93–0.67; p=0.22) in the Asia Pacific region, −0.56 (95% CI, 1.14–0.02; p=0.06) in the United States, and 0.32 (95% CI, 0.88–0.25; p=0.28) in Europe. However, the overall statistical heterogeneity was high (I2=94%).
The functional outcomes were improved in a comparison between RARP and LRP (Fig. 3). The urinary incontinence rate at 12 months was lower for RARP than for LRP (RR, 0.43; 95% CI, 0.31–0.60; p<0.000001), and statistical heterogeneity was low (I2=0%). The potency recovery rate was higher for RARP than for LRP at postoperative 12 months (RR, 1.38; 95% CI, 1.11–1.70; I2=78%; p=0.003). Potency recovery was defined as an International Index of Erectile Function 5 (IIEF-5)>17.
The overall positive surgical margin (PSM) results were investigated in 7 studies. When analyzing PSM rates in the pT2 group, the RARP and LRP series had PSM rates of 14.2% (123 of 864 cases) and 11.3% (97 of 860 cases) with an RR of 1.22 (95% CI, 0.98–1.54; p=0.11). In the pT3 group, the PSM rates for RARP and LRP were 43.1% (116 of 269 cases) and 34.4% (72 of 209 cases) with an RR of 1.26 (95% CI, 1.00–1.58; p=0.05) (Fig. 4).
The biochemical recurrence (BCR) rate was significantly lower for RARP than for LRP (RR, 0.59; 95% CI, 0.48–0.73; I2=0%; p<0.00001) (Fig. 4). Five studies used prostate-specific antigen (PSA)≥0.2 ng/mL to indicate BCR, 1 study used PSA≥0.4 ng/mL, and 2 studies did not define the cutoff used. The follow-up period ranged from 3 months to 60 months. The overall BCR rates for RARP and LRP were 8.6% (162 of 1,885 cases) and 13.7% (314 of 2,288 cases).
A funnel plot analysis for organ injury, blood transfusion rate, length of stay, potency, and overall BCR revealed a symmetrical funnel plot, indicating no publication bias (Fig. 5) (p=0.53, p=0.47, p=0.26, p=0.10, and p=0.70, respectively, Egger test). However, operative time revealed asymmetry on the funnel plot (p=0.01, Egger test).
Our study revealed three important findings: (1) the risk of complications after RARP was significantly lower than after LRP; (2) in comparison with LRP, the risk of urinary incontinence at 12 months was significantly lower and the potency rate was higher with RARP; (3) the BCR rate was significantly lower after RARP than after LRP.
The major findings of this meta-analysis showed significant differences in complications such as organ injury and other major complications according to the Clavien-Dindo (III–V) classification. The major complication rate was significantly lower for RARP than for LRP. Although both laparoscopic and robotic surgeries are regarded as minimally invasive techniques, the main advantages of RARP, such as seven degrees of freedom in robotic arm movement and magnified 3D vision of the robotic platform, result in decreased postoperative complications.
Incontinence is another complication of prostatectomy that greatly affects quality of life [5]. The urinary incontinence rate is influenced by the definition of incontinence. Thus, as reported previously, any systematic review is difficult to accomplish owing to the varying definitions used for urinary incontinence, such as involuntary urine loss [6], needing zero or 1 pad [7], and also those in the International Consultation on Incontinence Questionnaire-Short Form survey [8].
The urinary incontinence rate after 12 months was significantly lower with RARP than with LRP in this meta-analysis and in other randomized controlled trials, in which the rates for RARP and LRP were 6% and 17%, respectively, but without statistical significance owing to the limited study population [9]. Many modifications have been suggested in the field of robotics to improve the continence rate. Patel et al. [10] reported a peri-urethral suspension suture technique to improve the rate (92.8% vs. 83%; p=0.013 over 3 months). A modified posterior reconstruction that increased the continence rate by 4 weeks was reported by Coelho et al. [11]. In 2011, Asimakopoulos et al. [9] suggested a pubovesical-complex-sparing technique, in which a ventral plane was developed between the detrusor apron and the prostate. Owing to the technical feasibility of robotic assistance, reconstruction procedures have additionally improved the continence rate.
The potency recovery rate is another major concern for patients undergoing prostatectomy. The most common reason for failure to reach pentafecta is erectile dysfunction (35%) [12]. Our findings differed from those previously reported by NETSCC (2012), which showed no difference in the potency rate between RARP and LRP at 12 months postoperatively. In our meta-analysis, the potency rate of RARP at 12 months was significantly higher than that for LRP. Different potency recovery rates could be due to several factors, including different definitions of erectile dysfunction, various characteristics of the surgery, and differences in postsurgical rehabilitation [13]. Potency recovery was measured by using several methods, including the IIEF-5 and the Sexual Health Inventory for Men score. Various techniques for preserving potency have been developed on the robotic platform, including cavernous nerve preservation. Several nerve-sparing techniques were developed in previous studies. For example, Ahlering et al. [4] evaluated the adverse effects of electrocautery on dissection of the prostate and the superiority of cautery-free nerve-sparing techniques on the recovery of potency. Menon et al. [14] evaluated the “Veil of Aphrodite” technique in which the inter-fascial plane was extended towards the apex and laterally towards the prostatic pedicle. At 6 to 18 months postoperatively, 94% of men who attempted sexual intercourse after undergoing this technique reported success, with a median SHIM score of 18 out of 25. Following recent discoveries of the periprostatic fascial anatomy, extrafascial, interfascial, and intrafascial approaches have been developed. Comparing inter-fascial and extrafascial approaches, Shikanov et al. [15] reported a significantly improved potency rate (p=0.03) using the interfascial approach.
Three-dimensional magnified visualization of the robotic platform has enabled meticulous dissection of the periprostatic fascia layer and the neurovascular bundle. Further insights into the multilayered structure of the periprostatic fascia and the course of the cavernous nerves have supported the development of intra- or interfascial surgical planes, which have enabled improved functional outcomes in urinary incontinence and potency. A PSA level>0.2 ng/mL was selected as the important criterion, based on recommendations of clinical practice guidelines [16]. In an oncological RARP study, Menon et al. [17] reported biochemical-free survival rates of 95.1%, 90.6%, 86.6%, and 81.0% after 1, 3, 5, and 7 years, respectively. Few studies have reported BCR rates after RARP and LRP. Recently, Porpiglia et al. [18] reported BCR-free survival rates of 98% for a RARP group and 92.5% for an LRP group (p=0.190).
The oncologic outcome in the current study was noteworthy because of the statistically signif icant differences in BCR rates between the two groups. Ficarra et al. [13] demonstrated that BCR was significantly influenced by surgical experience, clinical tumor size, and anatomic tumor characteristics. Kim et al. [19] analyzed the preoperative predictors of BCR using multivariable analysis, which suggested that PSA, pathologic stage, pathologic Gleason score, and PSM were independently associated with BCR. In a Japanese study, the predictive factors of BCR following RARP were serum PSA levels, the percentage of positive cores, and the Gleason score. PSA density was also a strong predictor of advanced pathological features and BCR [20]. In our systematic review, the oncologic results for BCR showed a statistically significantly improved BCR in the RARP group relative to the LRP group, although the propensity score matching was similar between the groups. The PSM patients in the intermediate- and high-risk disease groups had higher rates of BCR than did those who were marginal or negative. However, the PSM in the low-risk disease group was not associated with disease progression [21]. After adjustment for differences in clinical and pathological features, the presence of a base margin was significantly associated with a shorter time to recurrence for intermediate- and high-risk disease. The apex margin also was associated with the time to recurrence, but not statistically so for intermediate-risk disease. Thus, the similar PSM rate did not indicate a similar BCR rate. The length of the PSM was also independently predictive of BCR. Patients with a PSM <1 mm appeared to have similar outcomes compared with those with negative surgical margins [22]. Patients with a PSM <1 mm did not differ from those with a negative margin, and as the length of the positive margin increased so did the risk of BCR. Interestingly, the risk of BCR did not differ between patients with a negative surgical margin and those with a PSM <1 mm.
The current study had several limitations. First, because this is a relatively new procedure, data were lacking on long-term oncologic results following RARP, such as the cancer-specific survival rate. Second, significant heterogeneity was evident in terms of surgical experience and definition of functional outcomes. Third, some of recently published articles had far larger cohorts, which strongly influenced the meta-analysis. Fourth, there was an era bias in several centers in that RARP was performed after LRP.
In conclusion, RARP showed favorable results compared with LRP. However, few long-term, high-quality studies are available comparing RARP and LRP. Although further studies are needed, our results revealed that RARP had an improved BCR rate, potency rate, and continence rate with fewer complications than LRP. Further high-quality studies that minimize confounding and selection biases with long-term follow-up are needed to further clarify the clinical efficacy and safety of RARP.
ACKNOWLEDGMENTS
This study was completed as part of the health technology assessment report (project No. NA 2013-007) funded by National Evidence-based Healthcare Collaborating Agency in South Korea.
References
1. Berge V, Berg RE, Hoff JR, Wessel N, Diep LM, Karlsen SJ, et al. A prospective study of transition from laparoscopic to robot-assisted radical prostatectomy: quality of life outcomes after 36-month follow-up. Urology. 2013; 81:781–786. PMID: 23465150.

2. Asimakopoulos AD, Miano R, Di Lorenzo N, Spera E, Vespasiani G, Mugnier C. Laparoscopic versus robot-assisted bilateral nerve-sparing radical prostatectomy: comparison of pentafecta rates for a single surgeon. Surg Endosc. 2013; 27:4297–4304. PMID: 23807752.

3. Lee YS, Han WK, Oh YT, Choi YD, Yang SC, Rha KH. Robot-assisted laparoscopic radical prostatectomy: four cases. Yonsei Med J. 2007; 48:341–346. PMID: 17461539.

4. Ahlering TE, Skarecky D, Borin J. Impact of cautery versus cautery-free preservation of neurovascular bundles on early return of potency. J Endourol. 2006; 20:586–589. PMID: 16903820.

5. Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008; 358:1250–1261. PMID: 18354103.

6. van der Poel HG, de Blok W, Joshi N, van Muilekom E. Preservation of lateral prostatic fascia is associated with urine continence after robotic-assisted prostatectomy. Eur Urol. 2009; 55:892–900. PMID: 19171418.

7. Borin JF, Skarecky DW, Narula N, Ahlering TE. Impact of urethral stump length on continence and positive surgical margins in robot-assisted laparoscopic prostatectomy. Urology. 2007; 70:173–177. PMID: 17656233.

8. Novara G, Ficarra V, D'elia C, Secco S, Cioffi A, Cavalleri S, et al. Evaluating urinary continence and preoperative predictors of urinary continence after robot assisted laparoscopic radical prostatectomy. J Urol. 2010; 184:1028–1033. PMID: 20643426.

9. Asimakopoulos AD, Pereira Fraga CT, Annino F, Pasqualetti P, Calado AA, Mugnier C. Randomized comparison between laparoscopic and robot-assisted nerve-sparing radical prostatectomy. J Sex Med. 2011; 8:1503–1512. PMID: 21324093.

10. Patel VR, Coelho RF, Palmer KJ, Rocco B. Periurethral suspension stitch during robot-assisted laparoscopic radical prostatectomy: description of the technique and continence outcomes. Eur Urol. 2009; 56:472–478. PMID: 19560260.

11. Coelho RF, Chauhan S, Orvieto MA, Sivaraman A, Palmer KJ, Coughlin G, et al. Influence of modified posterior reconstruction of the rhabdosphincter on early recovery of continence and anastomotic leakage rates after robot-assisted radical prostatectomy. Eur Urol. 2011; 59:72–80. PMID: 20801579.

12. Patel VR, Sivaraman A, Coelho RF, Chauhan S, Palmer KJ, Orvieto MA, et al. Pentafecta: a new concept for reporting outcomes of robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2011; 59:702–707. PMID: 21296482.

13. Ficarra V, Novara G, Fracalanza S, D'Elia C, Secco S, Iafrate M, et al. A prospective, non-randomized trial comparing robot-assisted laparoscopic and retropubic radical prostatectomy in one European institution. BJU Int. 2009; 104:534–539. PMID: 19281468.

14. Menon M, Shrivastava A, Bhandari M, Satyanarayana R, Siva S, Agarwal PK. Vattikuti Institute prostatectomy: technical modifications in 2009. Eur Urol. 2009; 56:89–96. PMID: 19403236.

15. Shikanov S, Woo J, Al-Ahmadie H, Katz MH, Zagaja GP, Shalhav AL, et al. Extrafascial versus interfascial nerve-sparing technique for robotic-assisted laparoscopic prostatectomy: comparison of functional outcomes and positive surgical margins characteristics. Urology. 2009; 74:611–616. PMID: 19616830.

16. Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014; 65:467–479. PMID: 24321502.

17. Menon M, Bhandari M, Gupta N, Lane Z, Peabody JO, Rogers CG, et al. Biochemical recurrence following robot-assisted radical prostatectomy: analysis of 1384 patients with a median 5-year follow-up. Eur Urol. 2010; 58:838–846. PMID: 20869162.

18. Porpiglia F, Morra I, Lucci Chiarissi M, Manfredi M, Mele F, Grande S, et al. Randomised controlled trial comparing laparoscopic and robot-assisted radical prostatectomy. Eur Urol. 2013; 63:606–614. PMID: 22840353.

19. Kim KH, Lim SK, Shin TY, Chung BH, Hong SJ, Rha KH. Biochemical outcomes after robot-assisted radical prostatectomy in patients with follow-up more than 5-years. Asian J Androl. 2013; 15:404–408. PMID: 23524532.

20. Hashimoto T, Yoshioka K, Gondo T, Ozu C, Horiguchi Y, Namiki K, et al. Preoperative prognostic factors for biochemical recurrence after robot-assisted radical prostatectomy in Japan. Int J Clin Oncol. 2014; 19:702–707. PMID: 24048883.

21. Choo MS, Cho SY, Ko K, Jeong CW, Lee SB, Ku JH, et al. Impact of positive surgical margins and their locations after radical prostatectomy: comparison of biochemical recurrence according to risk stratification and surgical modality. World J Urol. 2014; 32:1401–1409. PMID: 24362883.

22. Shikanov S, Song J, Royce C, Al-Ahmadie H, Zorn K, Steinberg G, et al. Length of positive surgical margin after radical prostatectomy as a predictor of biochemical recurrence. J Urol. 2009; 182:139–144. PMID: 19450829.

23. Bolenz C, Gupta A, Hotze T, Ho R, Cadeddu JA, Roehrborn CG, et al. Cost comparison of robotic, laparoscopic, and open radical prostatectomy for prostate cancer. Eur Urol. 2010; 57:453–458. PMID: 19931979.

24. Durand X, Vaessen C, Bitker MO, Richard F. Retropubic, laparoscopic and robot-assisted total prostatectomies: comparison of postoperative course and histological and functional results based on a series of 86 prostatectomies. Prog Urol. 2008; 18:60–67. PMID: 18342158.
25. Hakimi AA, Blitstein J, Feder M, Shapiro E, Ghavamian R. Direct comparison of surgical and functional outcomes of robotic-assisted versus pure laparoscopic radical prostatectomy: single-surgeon experience. Urology. 2009; 73:119–123. PMID: 18952268.

26. Ball AJ, Gambill B, Fabrizio MD, Davis JW, Given RW, Lynch DF, et al. Prospective longitudinal comparative study of early health-related quality-of-life outcomes in patients undergoing surgical treatment for localized prostate cancer: a short-term evaluation of five approaches from a single institution. J Endourol. 2006; 20:723–731. PMID: 17094746.
27. Drouin SJ, Vaessen C, Hupertan V, Comperat E, Misraï V, Haertig A, et al. Comparison of mid-term carcinologic control obtained after open, laparoscopic, and robot-assisted radical prostatectomy for localized prostate cancer. World J Urol. 2009; 27:599–605. PMID: 19421755.

28. Gosseine PN, Mangin P, Leclers F, Cormier L. Pure laparoscopic versus robotic-assisted laparoscopic radical prostatectomy: comparative study to assess functional urinary outcomes. Prog Urol. 2009; 19:611–617. PMID: 19800550.
29. Hu JC, Nelson RA, Wilson TG, Kawachi MH, Ramin SA, Lau C, et al. Perioperative complications of laparoscopic and robotic assisted laparoscopic radical prostatectomy. J Urol. 2006; 175:541–546. PMID: 16406991.

30. Joseph JV, Vicente I, Madeb R, Erturk E, Patel HR. Robot-assisted vs pure laparoscopic radical prostatectomy: are there any differences? BJU Int. 2005; 96:39–42. PMID: 15963117.

31. Rozet F, Jaffe J, Braud G, Harmon J, Cathelineau X, Barret E, et al. A direct comparison of robotic assisted versus pure laparoscopic radical prostatectomy: a single institution experience. J Urol. 2007; 178:478–482. PMID: 17561160.

32. Trabulsi EJ, Linden RA, Gomella LG, McGinnis DE, Strup SE, Lallas CD. The addition of robotic surgery to an established laparoscopic radical prostatectomy program: effect on positive surgical margins. Can J Urol. 2008; 15:3994–3999. PMID: 18405448.
33. Cho JW, Kim TH, Sung GT. Laparoscopic radical prostatectomy versus robot-assisted laparoscopic radical prostatectomy: a single surgeon's experience. Korean J Urol. 2009; 50:1198–1202.

34. Kasraeian A, Barret E, Chan J, Sanchez-Salas R, Validire P, Cathelineau X, et al. Comparison of the rate, location and size of positive surgical margins after laparoscopic and robot-assisted laparoscopic radical prostatectomy. BJU Int. 2011; 108:1174–1178. PMID: 21392221.

35. Kermarrec I, Mangin P, Koutlidis N, Mourey E, Cormier L. Does robotics improve laparoscopic radical prostatectomy in complex surgical cases? Prog Urol. 2010; 20:638–643. PMID: 20951932.
36. Koutlidis N, Mourey E, Champigneulle J, Mangin P, Cormier L. Robot-assisted or pure laparoscopic nerve-sparing radical prostatectomy: what is the optimal procedure for the surgical margins? A single center experience. Int J Urol. 2012; 19:1076–1081. PMID: 22860572.

37. Lee HW, Lee HM, Seo SI. Comparison of initial surgical outcomes between laparoscopic radical prostatectomy and robot-assisted laparoscopic radical prostatectomy performed by a single surgeon. Korean J Urol. 2009; 50:468–474.

38. Magheli A, Gonzalgo ML, Su LM, Guzzo TJ, Netto G, Humphreys EB, et al. Impact of surgical technique (open vs laparoscopic vs robotic-assisted) on pathological and biochemical outcomes following radical prostatectomy: an analysis using propensity score matching. BJU Int. 2011; 107:1956–1962. PMID: 21044243.

39. Nakamura LY, Nunez RN, Castle EP, Andrews PE, Humphreys MR. Different approaches to an inguinal hernia repair during a simultaneous robot-assisted radical prostatectomy. J Endourol. 2011; 25:621–624. PMID: 21355775.

40. Park B, Kim W, Jeong BC, Jeon SS, Lee HM, Choi HY, et al. Comparison of oncological and functional outcomes of pure versus robotic-assisted laparoscopic radical prostatectomy performed by a single surgeon. Scand J Urol. 2013; 47:10–18. PMID: 22835035.

41. Ploussard G, de la Taille A, Moulin M, Vordos D, Hoznek A, Abbou CC, et al. Comparisons of the perioperative, functional, and oncologic outcomes after robot-assisted versus pure extraperitoneal laparoscopic radical prostatectomy. Eur Urol. 2014; 65:610–619. PMID: 23245815.

42. Stolzenburg JU, Qazi HA, Holze S, Mende M, Nicolaus M, Franz T, et al. Evaluating the learning curve of experienced laparoscopic surgeons in robot-assisted radical prostatectomy. J Endourol. 2013; 27:80–85. PMID: 22834963.

43. Willis DL, Gonzalgo ML, Brotzman M, Feng Z, Trock B, Su LM. Comparison of outcomes between pure laparoscopic vs robot-assisted laparoscopic radical prostatectomy: a study of comparative effectiveness based upon validated quality of life outcomes. BJU Int. 2012; 109:898–905. PMID: 21933328.

44. Wolanski P, Chabert C, Jones L, Mullavey T, Walsh S, Gianduzzo T. Preliminary results of robot-assisted laparoscopic radical prostatectomy (RALP) after fellowship training and experience in laparoscopic radical prostatectomy (LRP). BJU Int. 2012; 110(Suppl 4):64–70. PMID: 23194128.

Fig. 2
Cumulative analyses of robot-assisted radical prostatectomy comparing laparoscopic radical prostatectomy in postoperative complication (A: Clavien-dindo classification, B: Organ injury). RARP, robot-assisted radical prostatectomy; LRP, laparoscopic radical prostatectomy; M-H, Mantel Haenszel; CI, confidence interval; df, degrees of freedom.
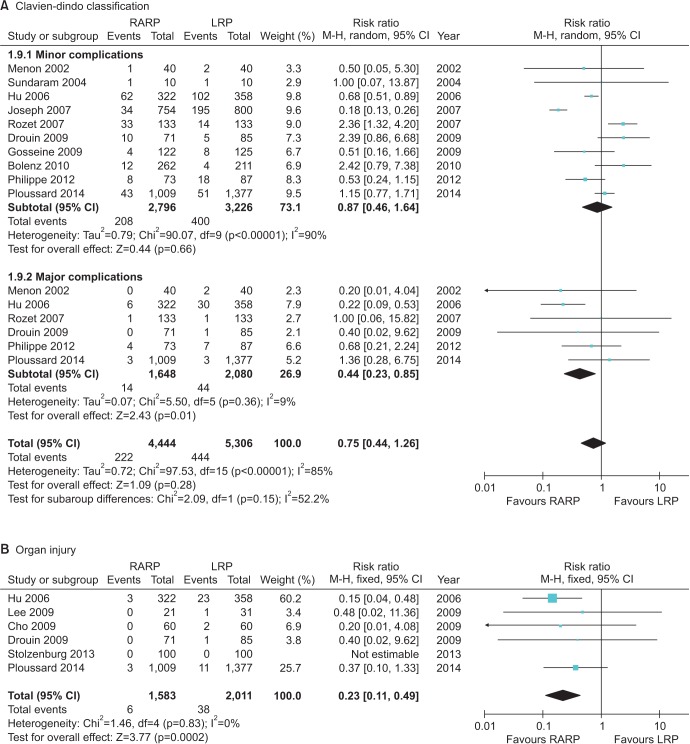
Fig. 3
Cumulative analyses of robot-assisted radical prostatectomy comparing laparoscopic radical prostatectomy in functional outcome (A: Urinary incontinence rate, B: Sexual function recovery rate). RARP, robot-assisted radical prostatectomy; LRP, laparoscopic radical prostatectomy; M-H, Mantel-Haenszel; CI, confidence interval; df, degrees of freedom.
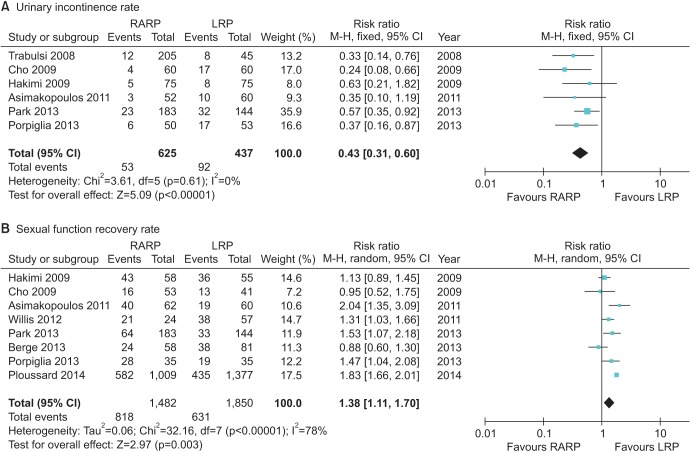
Fig. 4
Cumulative analyses of robot-assisted radical prostatectomy comparing laparoscopic radical prostatectomy in oncologic outcome (A: Positive surgical margin, B: Biochemical recurrence). RARP, robot-assisted radical prostatectomy; LRP, laparoscopic radical prostatectomy; M-H, Mantel-Haenszel; CI, confidence interval; df, degrees of freedom.
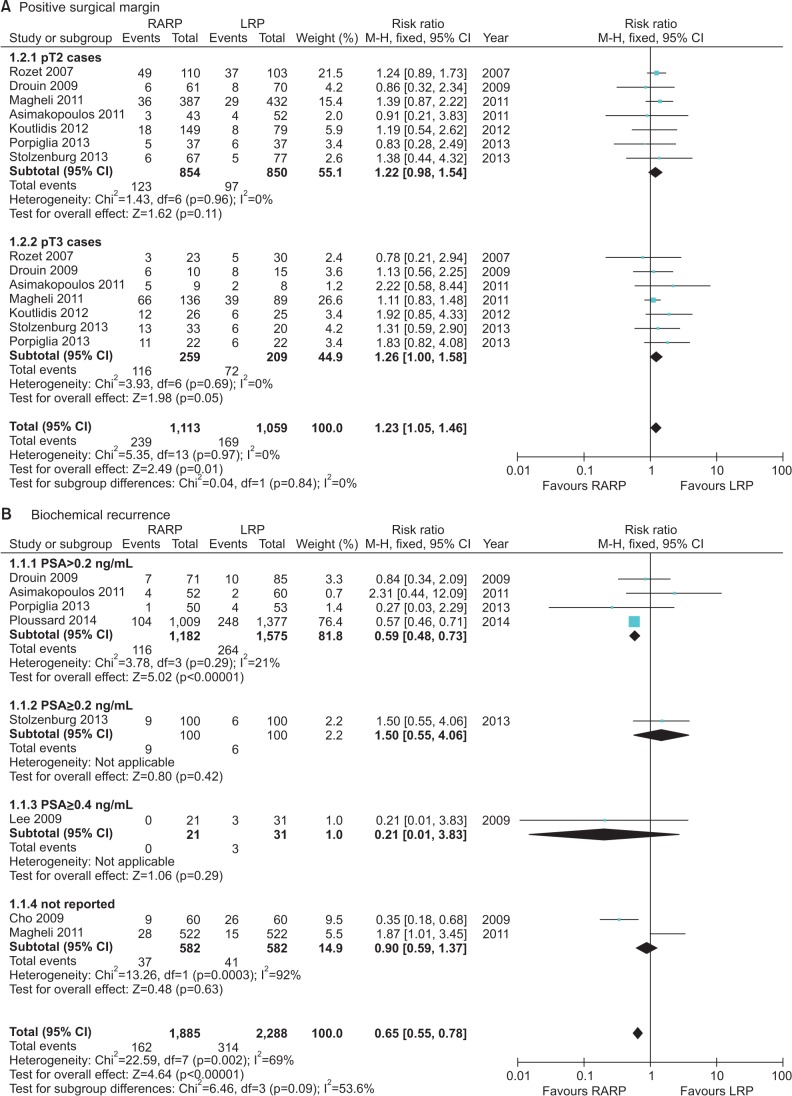
Fig. 5
Funnel plot of the studies of organ injury (A), blood transfusion rate (B), operative time (C), length of stay (D), potency (E) and overall biochemical recurrence (F) used in the meta-analysis.
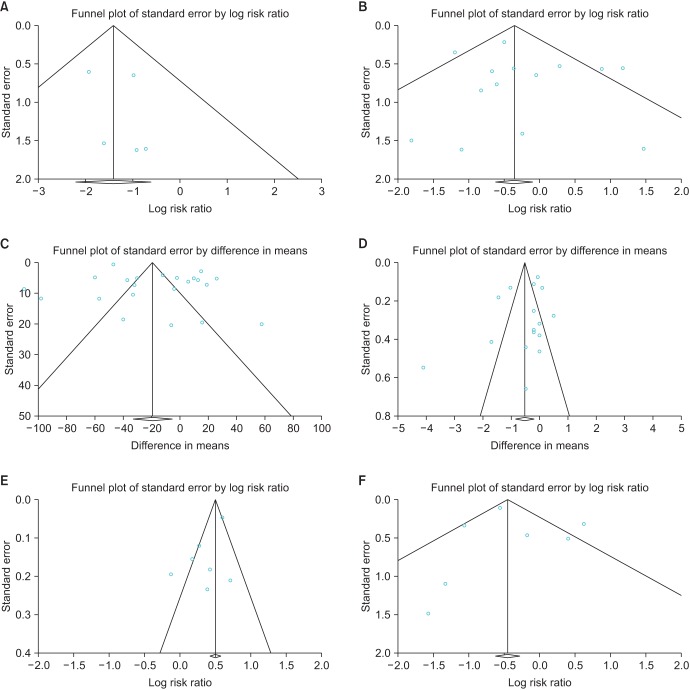
Table 1
The characteristics of the included studies
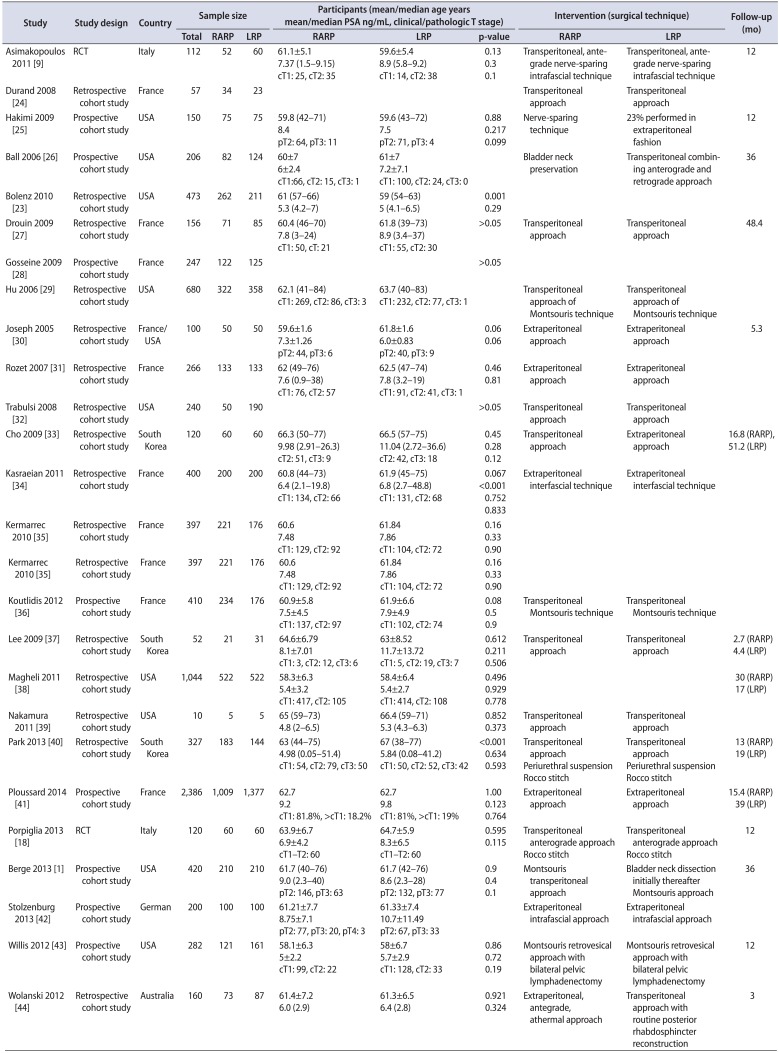
| Study | Study design | Country | Sample size | Participants (mean/median age years mean/median PSA ng/mL, clinical/pathologic T stage) | Intervention (surgical technique) | Follow-up (mo) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | RARP | LRP | RARP | LRP | p-value | RARP | LRP | ||||
| Asimakopoulos 2011 [9] | RCT | Italy | 112 | 52 | 60 | 61.1±5.1 | 59.6±5.4 | 0.13 | Transperitoneal, antegrade nerve-sparing intrafascial technique | Transperitoneal, antegrade nerve-sparing intrafascial technique | 12 |
| 7.37 (1.5–9.15) | 8.9 (5.8–9.2) | 0.3 | |||||||||
| cT1: 25, cT2: 35 | cT1: 14, cT2: 38 | 0.1 | |||||||||
| Durand 2008 [24] | Retrospective cohort study | France | 57 | 34 | 23 | Transperitoneal approach | Transperitoneal approach | ||||
| Hakimi 2009 [25] | Retrospective cohort study | USA | 150 | 75 | 75 | 59.8 (42–71) | 59.6 (43–72) | 0.88 | Nerve-sparing technique | 23% performed in extraperitoneal fashion | 12 |
| 8.4 | 7.5 | 0.217 | |||||||||
| pT2: 64, pT3: 11 | pT2: 71, pT3: 4 | 0.099 | |||||||||
| Ball 2006 [26] | Prospective cohort study | USA | 206 | 82 | 124 | 60±7 | 61±7 | Bladder neck preservation | Transperitoneal combining anterograde and retrograde approach | 36 | |
| 6±2.4 | 7.2±7.1 | ||||||||||
| cT1:66, cT2: 15, cT3: 1 | cT1: 100, cT2: 24, cT3: 0 | ||||||||||
| Bolenz 2010 [23] | Retrospective cohort study | USA | 473 | 262 | 211 | 61 (57–66) | 59 (54–63) | 0.001 | |||
| 5.3 (4.2–7) | 5 (4.1–6.5) | 0.29 | |||||||||
| Drouin 2009 [27] | Retrospective cohort study | France | 156 | 71 | 85 | 60.4 (46–70) | 61.8 (39–73) | >0.05 | Transperitoneal approach | Transperitoneal approach | 48.4 |
| 7.8 (3–24) | 8.9 (3.4–37) | ||||||||||
| cT1: 50, cT: 21 | cT1: 55, cT2: 30 | ||||||||||
| Gosseine 2009 [28] | Prospective cohort study | France | 247 | 122 | 125 | >0.05 | |||||
| Hu 2006 [29] | Retrospective cohort study | USA | 680 | 322 | 358 | 62.1 (41–84) | 63.7 (40–83) | Transperitoneal approach of Montsouris technique | Transperitoneal approach of Montsouris technique | ||
| cT1: 269, cT2: 86, cT3: 3 | cT1: 232, cT2: 77, cT3: 1 | ||||||||||
| Joseph 2005 [30] | Retrospective cohort study | France/USA | 100 | 50 | 50 | 59.6±1.6 | 61.8±1.6 | 0.06 | Extraperitoneal approach | Extraperitoneal approach | 5.3 |
| 7.3±1.26 | 6.0±0.83 | 0.06 | |||||||||
| pT2: 44, pT3: 6 | pT2: 40, pT3: 9 | ||||||||||
| Rozet 2007 [31] | Retrospective cohort study | France | 266 | 133 | 133 | 62 (49–76) | 62.5 (47–74) | 0.46 | Extraperitoneal approach | Extraperitoneal approach | |
| 7.6 (0.9–38) | 7.8 (3.2–19) | 0.81 | |||||||||
| cT1: 76, cT2: 57 | cT1: 91, cT2: 41, cT3: 1 | ||||||||||
| Trabulsi 2008 [32] | Retrospective cohort study | USA | 240 | 50 | 190 | >0.05 | Transperitoneal approach | Transperitoneal approach | |||
| Cho 2009 [33] | Retrospective cohort study | South Korea | 120 | 60 | 60 | 66.3 (50–77) | 66.5 (57–75) | 0.45 | Transperitoneal approach | Extraperitoneal approach | 16.8 (RARP), 51.2 (LRP) |
| 9.98 (2.91–26.3) | 11.04 (2.72–36.6) | 0.28 | |||||||||
| cT2: 51, cT3: 9 | cT2: 42, cT3: 18 | 0.12 | |||||||||
| Kasraeian 2011 [34] | Retrospective cohort study | France | 400 | 200 | 200 | 60.8 (44–73) | 61.9 (45–75) | 0.067 | Extraperitoneal interfascial technique | Extraperitoneal interfascial technique | |
| 6.4 (2.1–19.8) | 6.8 (2.7–48.8) | <0.001 | |||||||||
| cT1: 134, cT2: 66 | cT1: 131, cT2: 68 | 0.752 | |||||||||
| 0.833 | |||||||||||
| Kermarrec 2010 [35] | Retrospective cohort study | France | 397 | 221 | 176 | 60.6 | 61.84 | 0.16 | |||
| 7.48 | 7.86 | 0.33 | |||||||||
| cT1: 129, cT2: 92 | cT1: 104, cT2: 72 | 0.90 | |||||||||
| Kermarrec 2010 [35] | Retrospective cohort study | France | 397 | 221 | 176 | 60.6 | 61.84 | 0.16 | |||
| 7.48 | 7.86 | 0.33 | |||||||||
| cT1: 129, cT2: 92 | cT1: 104, cT2: 72 | 0.90 | |||||||||
| Koutlidis 2012 [36] | Prospective cohort study | France | 410 | 234 | 176 | 60.9±5.8 | 61.9±6.6 | 0.08 | Transperitoneal Montsouris technique | Transperitoneal Montsouris technique | |
| 7.5±4.5 | 7.9±4.9 | 0.5 | |||||||||
| cT1: 137, cT2: 97 | cT1: 102, cT2: 74 | 0.9 | |||||||||
| Lee 2009 [37] | Prospective cohort study | South Korea | 52 | 21 | 31 | 64.6±6.79 | 63±8.52 | 0.612 | Transperitoneal approach | Transperitoneal approach | 2.7 (RARP) |
| 8.1±7.01 | 11.7±13.72 | 0.211 | 4.4 (LRP) | ||||||||
| cT1: 3, cT2: 12, cT3: 6 | cT1: 5, cT2: 19, cT3: 7 | 0.506 | |||||||||
| Magheli 2011 [38] | Retrospective cohort study | USA | 1,044 | 522 | 522 | 58.3±6.3 | 58.4±6.4 | 0.496 | 30 (RARP) | ||
| 5.4±3.2 | 5.4±2.7 | 0.929 | 17 (LRP) | ||||||||
| cT1: 417, cT2: 105 | cT1: 414, cT2: 108 | 0.778 | |||||||||
| Nakamura 2011 [39] | Retrospective cohort study | USA | 10 | 5 | 5 | 65 (59–73) | 66.4 (59–71) | 0.852 | Transperitoneal approach | Transperitoneal approach | |
| 4.8 (2–6.5) | 5.3 (4.3–6.3) | 0.373 | |||||||||
| Park 2013 [40] | Retrospective cohort study | South Korea | 327 | 183 | 144 | 63 (44–75) | 67 (38–77) | <0.001 | Transperitoneal approach | Transperitoneal approach | 13 (RARP) |
| 4.98 (0.05–51.4) | 5.84 (0.08–41.2) | 0.634 | Periurethral suspension | Periurethral suspension | 19 (LRP) | ||||||
| cT1: 54, cT2: 79, cT3: 50 | cT1: 50, cT2: 52, cT3: 42 | 0.593 | Rocco stitch | Rocco stitch | |||||||
| Ploussard 2014 [41] | Prospective cohort study | France | 2,386 | 1,009 | 1,377 | 62.7 | 62.7 | 1.00 | Extraperitoneal approach | Extraperitoneal approach | 15.4 (RARP) |
| 9.2 | 9.8 | 0.123 | 39 (LRP) | ||||||||
| cT1: 81.8%, >cT1: 18.2% | cT1: 81%, >cT1: 19% | 0.764 | |||||||||
| Porpiglia 2013 [18] | RCT | Italy | 120 | 60 | 60 | 63.9±6.7 | 64.7±5.9 | 0.595 | Transperitoneal anterograde approach | Transperitoneal anterograde approach | 12 |
| 6.9±4.2 | 8.3±6.5 | 0.115 | |||||||||
| cT1–T2: 60 | cT1–T2: 60 | Rocco stitch | Rocco stitch | ||||||||
| Berge 2013 [1] | Prospective cohort study | USA | 420 | 210 | 210 | 61.7 (40–76) | 61.7 (42–76) | 0.9 | Montsouris transperitoneal approach | Bladder neck dissection initially thereafter Montsouris approach | 36 |
| 9.0 (2.3–40) | 8.6 (2.3–28) | 0.4 | |||||||||
| pT2: 146, pT3: 63 | pT2: 132, pT3: 77 | 0.1 | |||||||||
| Stolzenburg 2013 [42] | Prospective cohort study | German | 200 | 100 | 100 | 61.21±7.7 | 61.33±7.4 | Extraperitoneal intrafascial approach | Extraperitoneal intrafascial approach | ||
| 8.75±7.1 | 10.7±11.49 | ||||||||||
| pT2: 77, pT3: 20, pT4: 3 | pT2: 67, pT3: 33 | ||||||||||
| Willis 2012 [43] | Prospective cohort study | USA | 282 | 121 | 161 | 58.1±6.3 | 58±6.7 | 0.86 | Montsouris retrovesical approach with bilateral pelvic lymphadenectomy | Montsouris retrovesical approach with bilateral pelvic lymphadenectomy | 12 |
| 5±2.2 | 5.7±2.9 | 0.72 | |||||||||
| cT1: 99, cT2: 22 | cT1: 128, cT2: 33 | 0.19 | |||||||||
| Wolanski 2012 [44] | Retrospective cohort study | Australia | 160 | 73 | 87 | 61.4±7.2 | 61.3±6.5 | 0.921 | Extraperitoneal, antegrade, athermal approach | Transperitoneal approach with routine posterior rhabdosphincter reconstruction | 3 |
| 6.0 (2.9) | 6.4 (2.8) | 0.324 | |||||||||




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download