Abstract
Purpose
The fifth most common cancer is allocated to bladder cancer (BC) worldwide. Understanding the molecular mechanisms of BC invasion and metastasis to identify target therapeutic strategies will improve disease survival. So the aim of this study was to measure expression rate of zinc finger E-box binding homeobox 1 (ZEB1) and transforming growth factor-beta2 (TGF-β2) mRNA in tissue samples of patients with BC and its healthy adjacent tissue samples and their association with muscle invasion, size and grade of the tumor.
Materials and Methods
Tissue samples were collected from 35 newly diagnosed untreated patients with BC from 2013 to 2014. Total RNA was extracted from about 50-mg tissue samples using TRIzol reagent. TAKARA SYBR Premix EX Tag II was applied to determine the rate of mRNA expression by real-time polymerase chain reaction (PCR). To obtain final validation, PCR product of ZEB1 and TGF-β2 were sequenced. STATA 11 software was used to analyze the data.
Results
The expression level of ZEB1 in tumor samples was significantly more than of in healthy adjacent tissue samples. Up-regulation of TGF-β2 showed a strong association with muscle invasion (p=0.017). There was also demonstrated a relationship between over expression of ZEB1 with the tumor size (p=0.050).
Conclusions
It looks ZEB1 and TGF-β2 had a role in BC patients. In this study ZEB1 expression was higher in BC tissues than that of in healthy control tissues. There was demonstrated a markedly association between overexpression of TGF-β2 and muscle invasion. Therefore, they are supposed to be candidate as potential biomarkers for early detection and progression of BC.
The fifth most common cancer is allocated to bladder cancer (BC) worldwide [1]. In Iran, 3,764 new cases of BC were reported in 2010 [2]. BC resulted from gathering of genetic and epigenetic alterations including uncontrolled cellular proliferation, invasion, promoted cell survival and metastatic distribution [3].
Majority portion of BCs (80%) are diagnosed as nonmuscle invasive bladder cancers (NMIBCs) accompanied with rarely progression and good prognosis. The remaining cases (20%) are patients with muscle invasive bladder cancers (MIBCs) associated with poor prognosis due to high frequency of metastasis [4].
Cell motility increases through epithelial-mesenchymal transition (EMT) process which is suggested to play crucial role in cancer invasion and metastasis [5]. Loss of epithelial and the acquisition of mesenchymal features happens during EMT process [6]. Morphology and motility of the cells will change to gain mesenchymal features. Loss of E-cadherin, which is controlled by several transcriptional repressors, has been demonstrated as a significant primary event in bladder tumorigenesis which bind to the promoter of E-cadherin [7]. Up-regulation of transcriptional repressors such as zinc finger E-box binding homeobox 1 (ZEB 1), Snail and twist interfere in EMT process [8].
ZEB1 mRNA is targeted by human miR-141, miR-200a, miR-200b, and miR-200c and these miRNAs are down-regulated by transforming growth factor-beta (TGF-β) thus TGF-β up-regulates the expression level of ZEB1 [9] and activate EMT program [10].
On the other hand, different cell characteristics leading to metastasis could be regulated by TGF-β superfamily through over expression of matrix metalloproteinase-2 [11]. Mammalian TGF-β superfamily consists of three members (TGF-β1, TGF-β2 and TGF-β3) with similar structure and function [12].
It is shown an association between higher level of TGF-β2 in glioma tumors, advanced disease stage and poor prognosis [13].
Some studies indicated an over expression of ZEB1 in MIBCs compared to NMIBCs [41415] and some researchers reported expression level of TGF-β2 in colon cancer, glioma, gastric cancer and cervical lesions [12161718] but on our knowledge TGF-β2 assessed in an old study [6] as well as in concomitant with ZEB1 in bladder tumor and healthy tissue samples. Consequently, understanding the molecular mechanisms of BC progression is important to identify target therapeutic strategies and to improve disease survival [4] and prevent cancer progression [7].
The aim of this study was to evaluate TGF-β2 and ZEB1 mRNA expression in tissue samples of patients with BC and healthy adjacent tissue samples and their association with muscle invasion, grade and the size of the tumor.
This case-control study was conducted in School of Medicine, Hamadan University of Medical Sciences from 2013 to 2014. Thirty-five patients with newly diagnosed BC at the Beheshti and Bu-ali hospitals (Hamadan, Iran) enrolled in the study if they met the eligibility criteria. Study protocol approved by the Ethics Committee of the Hamadan University of Medical Sciences and Health (Hamadan, Iran) (approval number: 9112154556). All procedures have been carried out in accordance with Declaration of Helsinki and informed consent was obtained from all participants.
Patients who their disease clinically and pathologically confirmed irrespective of sex and age were evaluated. Transurethral resection tissue samples were collected from 35 newly diagnosed untreated patients with BC from 2013 to 2014. Control samples consisted of adjacent normal urothelium resected about 10 cm far from the tumor lesion of patients.
The following patients were excluded from the study: (1) patients with other organ or genitourinary cancers, (2) genitourinary infection, and (3) history of radiotherapy or chemotherapy.
After explaining the purpose of the study, written informed consent obtained from all participants. Then, cystoscopy and clear description of tumor done by an expert urologist under spinal anesthesia. The entire tumor was resected as deep as possible. After that, bladder and urethra were washed with normal saline three times, and then a normal urothelium area sample was taken at least 10 cm far from tumor bed. Immediately, the samples were washed with RNase-free cold saline solution, snap-frozen in liquid nitrogen and stored at -80℃ until pathologic confirmation and further analysis. All samples, observed by 2 pathologists, diagnosed transitional cell carcinomas with the proportion of tumor cells greater than 80%. The International Union against Cancer, World Health Organization/International Society of Urological Pathology criteria of 2004 was applied to assess tumor staging and histological grading, respectively.
Total RNA was extracted from 50 mg of tissue sample using TRIZOL Reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's protocol. Depending on the quantity of the precipitation, the extracted RNA was dissolved in 20- to 50-µL RNase-free water. Concentration and purity of total RNA was determined by optical density measurement using a Nano-Drop spectrophotometer (BioTeK, Winooski, VT, USA). Integrity of extracted RNA was evaluated by agarose electrophoresis.
Five microgram of total RNA was applied to synthesize cDNA using Reverse Transcription kit (Fermentas, Waltham, MA, USA). Quantification of mRNA expression level was performed by applying TAKARA SYBR Premix EX Tag II in a CFX96 real-time polymerase chain reaction (PCR) detection system (Bio-Rad, Hercules, CA, USA) in duplicate. Melting curve analysis was performed to evaluate specificity of primers. To design TGF-β2 mRNA and ZEB1 mRNA specific primers, we applied allele ID6 software and the designed primers was synthesized by Bioneer company (Daejeon, Korea) (Table 1) [19].
ZEB1 mRNA and TGF-β2 mRNA expression was normalized to 18s rRNA as internal control which there was no difference between case and control groups. No template control was included in each PCR run to evaluate contaminations which all were negative. Relative expression of the studied genes were calculated by the 2-ΔΔct method [20]. Findings greater and less than 1 were determined to classify over expression and lower expression, respectively. In order to final validation, sequencing of PCR products for ZEB1 and TGF-β2 were applied.
Seventy tissue samples from 35 patients with BC evaluated in present study (35 from malignant site and 35 from adjacent normal tissue). Two in the 35 patients were female and the remaining 33 were male. Mean age of patients was 71±11 (a range of 44 to 91 years old). In pathological examination, 63% of cases had muscle invasion categorized as MIBC, the rest was NMIBC. Sixteen of BC were low grade carcinoma as well as 17 were high-grade carcinoma. The 2 remaining tumor specimens were papillary urothelial neoplasm of low malignant potential.
There was indicated significant up-regulated ZEB1 mRNA in tumor tissue compared with healthy adjacent tissue (p=0.042) (Table 2). Interestingly, it was observed that ZEB1 mRNA was overexpressed in 57% of malignant tissue samples. The folding change of ZEB1 mRNA expressions was 2.19 in cancer group compared with control group. No relationship showed between TGF-β2 mRNA expression and BC (p=0.499) (Table 2).
It was not observed significant association between ZEB1 mRNA and TGF-β2 mRNA expression and smoking state (p=0.749 and p=0.599, respectively) (Table 3).
There was demonstrated higher expression level of TGF-β2 in MIBC group compared to NMIBC (p=0.001). The expression level of ZEB1 in MIBC group was higher than that of ZEB1 in NMIBC group. However, it was statistically non-significant (p=0.073). Mean standard deviation of ZEB1 and TGF-β2 mRNA is presented in Table 4. As indicated in Table 3, up-regulation of TGF-β2 showed a strong association with muscle invasion.
In tumor specimens, we did not see any significant association between grading and ZEB1 mRNA and TGF-β2 mRNA deregulation (p=0.363 and p=0.127, respectively). However, in according to CT of the mRNA target – CT of the reference gene in case and control group, separately (ΔCT) values, in a parallel direction with the poorly differentiation of tumor cells, we observed higher expression of TGF-β2 and ZEB1 (Table 5).
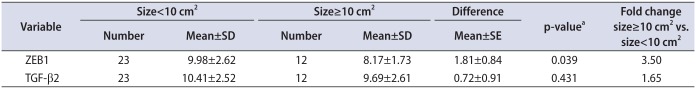
The tumor samples was subdivided based on the surface of the tumor, which was reported in 2 dimensional ultrasonography by radiologist, into greater and less than 10 cm2, the expression of ZEB1 mRNA was 3.5 fold higher in tumor samples with a size ≥10 cm2 compared to tumor samples with a size of <10 cm2 (p=0.039) (Table 6). In spite of 1.65 fold higher expression of TGF-β2 in tumor with size ≥10 cm2, we did not obtain statistically significant relationship between tumor size and TGF-β2 up-regulation (p=0.431) (Table 6).
BC has high mortality and 70% recurrence rate. More over it is the ninth prevalent and the second most common genitourinary tract malignant tumor [2122].
Early detection, prevention and treatment of cancer are very important. In this study we evaluated TGF-β2 and ZEB1 mRNA expression in BC and normal adjacent tissue and their relationship with muscle invasion, grade and the size of the tumor.
The level of TGF-β2 expression was not statistical significant between BC and control group. Normal tissue samples obtained from healthy adjacent tissue therefore; field effect may be a potential confounding variable in present study [23]. It was observed a 6.19 times up regulation of TGF-β2 in MIBC. The expression of TGF-β2 was not related to tumor size.
Our data supported by Gupta et al. [24] research which they confirmed the role of TGF-β in BC cell invasion. In a study, opposite to our results, expression of TGF-β2 in bladder tumor epithelial cells was higher than that of in normal epithelial cells [6].
Ma et al. [16] showed a higher expression of TGF-β2 in patients with early and advanced cancers compared to controls and it was detected a correlation between TGF-β2 mRNA expression and prognosis.
TGF-β2 mRNA and protein expression were significantly increased during colon carcinoma progression [12]. Gupta et al. [24] also showed over expression of TGF-β isoforms in invasive high-grade BC. In our study TGF-β2 expression was increasing as tumor grade increased. TGF-β2 also was diagnosed as a potential biomarker in cervical intraepithelial neoplasia [18]. In according to our findings TGF-β2 could be considered as a potential progression biomarker in BC.
The results showed signif icant ZEB1 mRNA up-regulation in case group. Although ZEB1 expression in MIBC group was 2.73 times higher than that of in NMIBC group but this difference was not significant. The expression also was greater in tumor samples with a size of ≥10 cm2.
In agree with this study, it was shown over expressed ZEB1 mRNA has been related to invasion in cancer patients [4].
Another study evaluated ZEB1 expression in bladder tumorigenesis and showed slightly low level ZEB1 expression in NMIBC and grades I/II compared to MIBC and tumor grade III respectively. They also observed no significant correlation, between tumor stage and grade, nodal involvement, vascular invasion, metastasis and survival [14]. It was not detected any significant association between ZEB1 expression, muscle invasion and grade of BC in our study.
Another study evaluated ZEB1 expression in noninvasive and invasive bladder tumor tissue samples. They reported ZEB1 was more frequently detected in high grade than in low-grade cancers [15]. In spite of higher expression in high-grade samples, significant over expressed ZEB1 did not obtain in present study. However; ZEB1 was up regulated in tumor tissues generally.
Remarkable higher expression of ZEB1 associated with tumor size has not been reported in other investigations.
There were some limitations in our research including: small study population, which future studies with larger sample size should be suggested. The next, in this study tissue samples were obtained using an invasive procedure. It is very helpful to design future studies based on non invasive biologic urine or serum samples to discover biomarkers. The last, grossly normal mucosa may has characteristics of tumor cells especially at molecular level and plays as confounding variable; therefore it would be better to design two control groups and introduce both normal tissue and healthy adjacent tissue samples as control group in such investigations.
Other studies should be done with large sample size in the future to evaluate the relation between ZEB1 and clinical follow-up and treatment out come.
ZEB1 and TGF-β2 play a role in BC. In this study expression of ZEB1 was higher in BC tissues than that of in healthy control tissues. There was demonstrated a markedly association between overexpression of TGF-β2 and muscle invasion. Therefore; they could be supposed to be potential biomarkers for early detection and progression of BC. Further studies should be performed about ZEB1 and TGF-β2 alterations in BC to understand role of these genes in tumor initiation, progression and metastasis.
ACKNOWLEDGMENTS
This study was funded by the Vic-chancellor of Research and Technology, Hamadan University of Medical Sciences.
References
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012; 62:10–29. PMID: 22237781.

2. Mousavi SM, et al. Guideline: National cancer registry (persian). 2nd ed. Tehran (IR): Ministry of health and medical education,office of Deputy minister for health, center for disease control and prevention,cancer office;2012.
3. Adam L, Zhong M, Choi W, Qi W, Nicoloso M, Arora A, et al. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin Cancer Res. 2009; 15:5060–5072. PMID: 19671845.

4. Wu K, Fan J, Zhang L, Ning Z, Zeng J, Zhou J, et al. PI3K/Akt to GSK3β/β-catenin signaling cascade coordinates cell colonization for bladder cancer bone metastasis through regulating ZEB1 transcription. Cell Signal. 2012; 24:2273–2282. PMID: 22906492.

5. Wu K, Ning Z, Zeng J, Fan J, Zhou J, Zhang T, et al. Silibinin inhibits β-catenin/ZEB1 signaling and suppresses bladder cancer metastasis via dual-blocking epithelial-mesenchymal transition and stemness. Cell Signal. 2013; 25:2625–2633. PMID: 24012496.

6. Eder IE, Stenzl A, Hobisch A, Cronauer MV, Bartsch G, Klocker H. Expression of transforming growth factors beta-1, beta 2 and beta 3 in human bladder carcinomas. Br J Cancer. 1997; 75:1753–1760. PMID: 9192977.

7. Matsui Y, Assi K, Ogawa O, Raven PA, Dedhar S, Gleave ME, et al. The importance of integrin-linked kinase in the regulation of bladder cancer invasion. Int J Cancer. 2012; 130:521–531. PMID: 21351095.

8. Shan Y, Zhang L, Bao Y, Li B, He C, Gao M, et al. Epithelial-mesenchymal transition, a novel target of sulforaphane via COX-2/MMP2, 9/Snail, ZEB1 and miR-200c/ZEB1 pathways in human bladder cancer cells. J Nutr Biochem. 2013; 24:1062–1069. PMID: 23159064.

9. Katoh Y, Katoh M. Hedgehog signaling, epithelial-to-mesenchymal transition and miRNA (review). Int J Mol Med. 2008; 22:271–275. PMID: 18698484.

10. Hurt EM, Saykally JN, Anose BM, Kalli KR, Sanders MM. Expression of the ZEB1 (deltaEF1) transcription factor in human: additional insights. Mol Cell Biochem. 2008; 318:89–99. PMID: 18622689.
11. Dehnavi E, Soheili ZS, Samiei S, Ataei Z, Aryan H. The effect of TGF-beta2 on MMP-2 production and activity in highly metastatic human bladder carcinoma cell line 5637. Cancer Invest. 2009; 27:568–574. PMID: 19219652.
12. Bellone G, Carbone A, Tibaudi D, Mauri F, Ferrero I, Smirne C, et al. Differential expression of transforming growth factors-beta1, -beta2 and -beta3 in human colon carcinoma. Eur J Cancer. 2001; 37:224–233. PMID: 11166150.
13. Hau P, Jachimczak P, Schlaier J, Bogdahn U. TGF-β2 signaling in high-grade gliomas. Curr Pharm Biotechnol. 2011; 12:2150–2157. PMID: 21619538.
14. Kenney PA, Wszolek MF, Rieger-Christ KM, Neto BS, Gould JJ, Harty NJ, et al. Novel ZEB1 expression in bladder tumorigenesis. BJU Int. 2011; 107:656–663. PMID: 20735391.

15. Lee H, Jun SY, Lee YS, Lee HJ, Lee WS, Park CS. Expression of miRNAs and ZEB1 and ZEB2 correlates with histopathological grade in papillary urothelial tumors of the urinary bladder. Virchows Arch. 2014; 464:213–220. PMID: 24306957.

16. Ma GF, Miao Q, Zeng XQ, Luo TC, Ma LL, Liu YM, et al. Transforming growth factor-β1 and -β2 in gastric precancer and cancer and roles in tumor-cell interactions with peripheral blood mononuclear cells in vitro. PLoS One. 2013; 8:e54249. PMID: 23342108.

17. Qiu B, Zhang D, Wang C, Tao J, Tie X, Qiao Y, et al. IL-10 and TGF-β2 are overexpressed in tumor spheres cultured from human gliomas. Mol Biol Rep. 2011; 38:3585–3591. PMID: 21088899.

18. Xu XC, Mitchell MF, Silva E, Jetten A, Lotan R. Decreased expression of retinoic acid receptors, transforming growth factor beta, involucrin, and cornifin in cervical intraepithelial neoplasia. Clin Cancer Res. 1999; 5:1503–1508. PMID: 10389939.
19. Schmittgen TD, Zakrajsek BA. Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J Biochem Biophys Methods. 2000; 46:69–81. PMID: 11086195.

20. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008; 3:1101–1108. PMID: 18546601.

21. Yoshino H, Enokida H, Chiyomaru T, Tatarano S, Hidaka H, Yamasaki T, et al. Tumor suppressive microRNA-1 mediated novel apoptosis pathways through direct inhibition of splicing factor serine/arginine-rich 9 (SRSF9/SRp30c) in bladder cancer. Biochem Biophys Res Commun. 2012; 417:588–593. PMID: 22178073.

22. Shirodkar SP, Lokeshwar VB. Potential new urinary markers in the early detection of bladder cancer. Curr Opin Urol. 2009; 19:488–493. PMID: 19584734.

23. Jones TD, Wang M, Eble JN, MacLennan GT, Lopez-Beltran A, Zhang S, et al. Molecular evidence supporting field effect in urothelial carcinogenesis. Clin Cancer Res. 2005; 11:6512–6519. PMID: 16166427.

24. Gupta S, Hau AM, Al-Ahmadie HA, Harwalkar J, Shoskes AC, Elson P, et al. Transforming growth factor-β is an upstream regulator of mammalian target of rapamycin complex 2-dependent bladder cancer cell migration and invasion. Am J Pathol. 2016; 186:1351–1360. PMID: 26988652.

Table 1
Characteristics of TGF-β2 and ZEB1 mRNA primers designed with allele ID6 software
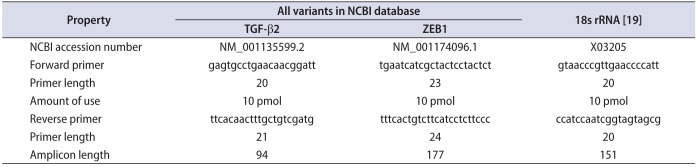
| Property | All variants in NCBI database | 18s rRNA [19] | |
|---|---|---|---|
| TGF-β2 | ZEB1 | ||
| NCBI accession number | NM_001135599.2 | NM_001174096.1 | X03205 |
| Forward primer | gagtgcctgaacaacggatt | tgaatcatcgctactcctactct | gtaacccgttgaaccccatt |
| Primer length | 20 | 23 | 20 |
| Amount of use | 10 pmol | 10 pmol | 10 pmol |
| Reverse primer | ttcacaactttgctgtcgatg | tttcactgtcttcatcctcttccc | ccatccaatcggtagtagcg |
| Primer length | 21 | 24 | 20 |
| Amplicon length | 94 | 177 | 151 |
Table 2
ΔCT values of ZEB1 and TGF-β2 between case and control groups
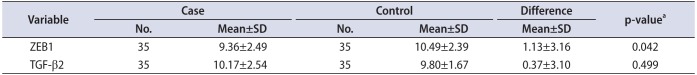
| Variable | Case | Control | Difference | p-valuea | ||
|---|---|---|---|---|---|---|
| No. | Mean±SD | No. | Mean±SD | Mean±SD | ||
| ZEB1 | 35 | 9.36±2.49 | 35 | 10.49±2.39 | 1.13±3.16 | 0.042 |
| TGF-β2 | 35 | 10.17±2.54 | 35 | 9.80±1.67 | 0.37±3.10 | 0.499 |
Table 3
Expression state of ZEB1 and TGF-β2 in according to smoking
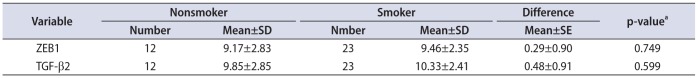
| Variable | Nonsmoker | Smoker | Difference | p-valuea | ||
|---|---|---|---|---|---|---|
| Number | Mean±SD | Nmber | Mean±SD | Mean±SE | ||
| ZEB1 | 12 | 9.17±2.83 | 23 | 9.46±2.35 | 0.29±0.90 | 0.749 |
| TGF-β2 | 12 | 9.85±2.85 | 23 | 10.33±2.41 | 0.48±0.91 | 0.599 |
Table 4
ΔCT values and relative quantification between nonmuscle invasive bladder cancer and muscle invasive bladder cancer
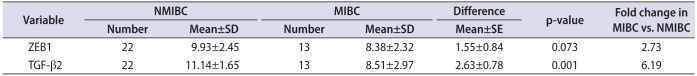
Table 5
Association between grading and ZEB1 and TGF-β2 expression
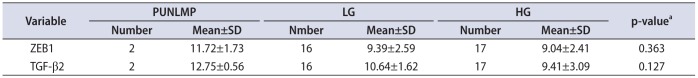
| Variable | PUNLMP | LG | HG | p-valuea | |||
|---|---|---|---|---|---|---|---|
| Number | Mean±SD | Number | Mean±SD | Number | Mean±SD | ||
| ZEB1 | 2 | 11.72±1.73 | 16 | 9.39±2.59 | 17 | 9.04±2.41 | 0.363 |
| TGF-β2 | 2 | 12.75±0.56 | 16 | 10.64±1.62 | 17 | 9.41±3.09 | 0.127 |
Table 6
ZEB1 and TGF-β2 mRNA expression and relative quantification between tumors with size<10 cm2 and size≥10 cm2




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download