Abstract
Purpose
To report the effects of combined onabotulinumtoxinA (Botox) injections and myofascial release physical therapy on myofascial pelvic pain (MFPP) by comparing pre- and posttreatment average pelvic pain scores, trigger points, and patient self-reported pelvic pain. Secondary outcomes were to examine posttreatment complications and determine demographic differences between patients with/without an improvement in pain.
Materials and Methods
This was an Institutional Review Board approved retrospective case series on women over 18 years with MFPP who received Botox and physical therapy between July 2006 and November 2014. Presence of trigger points and pelvic pain scores were determined by digital palpation of the iliococcygeus, puborectalis, obturator internus, and rectus muscles. Average pelvic pain scores (0–10) reflected an average of the scores obtained from palpation of each muscle. Self-reported improvement in pain was recorded as yes/no.
Results
Fifty women met the inclusion/exclusion criteria. Posttreatment, patients had lower average pelvic pain scores (3.7±4.0 vs. 6.4±1.8, p=0.005), and fewer trigger points (44% vs. 100%, p<0.001). Fifty-eight percent of patients (95% confidence interval, 44–72) noted an improvement in self-reported pain. Patients most likely to report no improvement in pain had chronic bowel disorders, while those most likely to report an improvement in pain had a history of past incontinence sling (p=0.03). Posttreatment complications included: constipation (8%), worsening urinary retention (2%), and urinary tract infection (4%).
Chronic pelvic pain affects approximately 15% of women aged 18–50 years [1]. Myofascial pelvic pain (MFPP) is a major contributor of chronic pelvic pain and is characterized by the presence of myofascial trigger points, or taught bands of muscle, in addition to regional pelvic pain [12]. The pathogenesis of MFPP is complex and occurs as a result of several interacting mechanisms [2]. Fundamentally, it is thought to be caused by an abnormal increase in acetylcholine release at the motor endplate nerve terminal, which causes sustained muscle fiber contractions. These sustained contractions in turn cause local muscle ischemia and pain [2].
Treatment options for MFPP are varied, ranging from physical therapy to local trigger point injections. Pelvic myofascial release physical therapy is often recommended as first line therapy in MFPP [3]. For patients who fail physical therapy, pharmacological treatment of their myofascial pain is an option [23]. However, despite compliance with these medications, some patients may continue to experience symptoms. Furthermore, other patients may not be compliant with the medications due to intolerable side effects. One promising alternative is treatment with onabotulinumtoxinA (Botox), which prevents the local release of acetylcholine at the motor end plates [4]. Only a few studies with small sample sizes have examined the effectiveness of Botox in the treatment of MFPP [5]. The concurrent use of Botox and physical therapy in the treatment of MFPP has not been formally studied.
The primary aims of this study were to report the effects of Botox injections combined with myofascial soft tissue release physical therapy (performed under general anesthesia) on a small case series of women with MFPP by comparing pre- and posttreatment average pelvic pain scores, trigger points, and patient self-reported pelvic pain. The secondary aims of this study were to report differences in patient characteristics and presenting pain symptoms between patients who reported an improvement versus no improvement in pelvic pain after undergoing treatment.
All patients over the age of 18 years who underwent treatment of their myofascial pain symptoms with Botox and physical therapy concurrently under general anesthesia at Tampa General Hospital between July 2006 and November 2014 were included in this retrospective case series. After approval was obtained from the Institutional Review Board (approval number: Pro00006405), patients were identified using the following International Classification of Diseases, 9th revision (ICD-9) procedure codes: physical therapy (93.39, 93.27, 93.21, 93.38) and Botox administration (99.57, 99.29, 83.98). Electronic medical records including clinic notes, anesthesia records, procedure notes, inpatient posttreatment notes and discharge summaries were reviewed.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The data reported in this study was retrospectively gathered. For this type of study formal consent is not required. This article does not contain any studies with animals performed by any of the authors.
Inclusion criteria for the study included women with chronic pelvic pain, pelvic floor trigger points on exam, and failure (with subsequent discontinuation) of at least one treatment modality including outpatient physical therapy and/or oral analgesics. Patients who underwent a concomitant procedure or were lost to follow-up were excluded. Data extracted included patient demographics, past medical history (including medical problems and previous surgeries), symptoms, pre- and posttreatment pelvic floor examination, and posttreatment course. Symptoms that patients complained of in addition to generalized pelvic pain (such as dyspareunia, muscle spasm, and suprapubic, coccyx, and rectal pain) were also recorded. Assessments of trigger points, Botox injections and physical therapy were performed by either a urogynecology attending or fellow under direct supervision by the attending. One attending received training by pelvic floor physical therapists during fellowship. The other attending is board certified in osteopathic medicine and was also trained in the same physical therapy techniques by the other attending.
To objectify patients' pelvic pain, each individual iliococcygeus, puborectalis, obturator internus, and anterior rectus muscle was digitally palpated bilaterally pre- and posttreatment. The presence of trigger points was noted when a contracted pelvic floor muscle revealed pain on palpation. Patients were also asked to verbally quantify their pain using a score of 0 to 10, with zero being no pain and 10 being equivalent to the worst possible pain ever experienced. For each patient, pain scores were averaged across all the muscles, and a pelvic pain score was assigned both pre- and posttreatment. Self-reported improvement in pain was further recorded as yes/no, and when there was no improvement, the type of pelvic pain was noted.
Botox with physical therapy procedures were performed in the operating room with patients under general anesthesia in the lithotomy position. Two hundred units of diluted onabotulinumtoxinA (Botox) were prepared using 20 mL of sterile, preservative-free, normal saline, to achieve a concentration of 10 units per mL. When patients had trigger points present in the rectus muscles, a separate concentration of 100 units of Botox was mixed with 20 mL of normal saline to achieve a concentration of 5 units per mL. Contracted pelvic floor muscles were identified on digital vaginal exam. A standard 20-gauge needle from a pudendal block kit was used to inject the Botox solution followed by 10–20 mL of 0.25% Marcaine into the identified trigger points. Multiple areas of the perineum were injected. Soft tissue myofascial release physical therapy (massaging and stretching of the myofascia that releases pressure and tightness of the muscles) was then performed transvaginally by the physician for 10–15 minutes. An additional 10–15 minutes of physical therapy was performed on the rectus muscles as need for trigger points in that region. Posttreatment, patients were seen in the 2 to 8 weeks after surgery. During that postoperative visit a repeat pelvic exam with palpation of each muscle and quantification of pain was performed. The physician who performed the surgery was not always the physician who examined the patient postoperatively.
Baseline demographic and clinical characteristics are summarized as mean and standard deviation, and percentages for categorical variables. These characteristics were compared using the Student t-test for continuous variables, and McNemar or Fisher exact test tests for categorical variables. All statistical tests were performed using Stata 13.1 (StataCorp LP., College Station, TX, USA) [6].
Of the 160 women who underwent treatment of MFPP with physical therapy and Botox at our institution, 100 had concomitant procedures performed (hysterectomy, salpingo-oophorectomy, incontinence sling revision/removal, placement of incontinence sling, vaginal mesh revision/removal, uterosacral ligment suspension, colpopexy, anterior/posterior repair, colporrhaphy, perineoplasty) and 10 were lost to follow-up, leaving 50 women who met our inclusion/exclusion criteria. Baseline characteristics are represented in Table 1. Mean age was 44.5 (standard deviation, ±15.2) years, body mass index 26.4±6.2 kg/m2, and parity 1.6±1.4. Most patients were white (90%), half were postmenopausal, and less than half (42%) had stage 1 pelvic support. The primary pain complaint for all women (100%) was dyspareunia. Most women (70%) had not undergone a prior pelvic reconstructive surgery. Of the women who did undergo prior pelvic surgery, most (14%) underwent a procedure in which mesh was placed including mesh for anterior repair, posterior repair, or incontinence sling.
There was a significant difference between pre- (6.4±1.8) and posttreatment (3.7±4.0) average pelvic pain scores for all patients (p=0.005). Posttreatment, there were significantly fewer patients with trigger points on pelvic examination (44% vs. 100%, p<0.001). Overall, 58% of patients (95% confidence interval, 44%–72%) noted an improvement in self-reported pelvic pain. Patients with and without an improvement in pain were not significantly different in terms of: presenting pain symptoms, severity and length of time with pain, and menopausal status (Table 2). Patients with chronic bowel disorders such as inflammatory bowel disease, irritable bowel disease, and diverticulosis were more likely to report no improvement (28.6%) versus an improvement (3.5%) in pain (p=0.03). Patients with previous placement of incontinence sling were more likely to report an improvement (20.7%) versus no improvement (0%) in pain (p=0.03).
Posttreatment, patients were followed for a mean of 6 weeks (range, 2–192 weeks). A total of 6 participants (12%) had a posttreatment complication including: constipation (8%), worsening of baseline urinary retention that did not resolve until placement of Interstim device (2%), and urinary tract infection (4%). Five patients (10%) underwent retreatment with another course of Botox and soft tissue myofascial release physical therapy.
Results from this retrospective case series suggest that Botox combined with soft tissue myofascial release physical therapy under anesthesia can be used as a treatment option for women with chronic pelvic pain secondary to MFPP. Patients who underwent treatment with Botox and physical therapy had significantly lower average pelvic pain scores and fewer posttreatment pelvic trigger points. Additionally, an improvement in self-reported pelvic pain was noted in more than half of participants, although this change was not statistically significant. To our knowledge, this is the first study to report the combined use of Botox and pelvic floor physical therapy in the treatment of pelvic pain secondary to MFPP.
As previously stated, the underlying etiology of myofascial pain is contracture of the pelvic floor muscles, or trigger points, which result in local tissue ischemia. Muscle pain and upward regulation of acetylcholine receptors occur as a result of the ischemia, which results in further formation of trigger points and more pain [789]. Physical therapy and, most recently, Botox have evolved as treatment modalities for MFPP because they focus on decreasing the presence of trigger points, as they are the underlying cause of the pain [1011].
The symptom-free interval provided by treatment with Botox or physical therapy for MFPP is not well established. Thiele, who was the first to describe the use of physical therapy in the treatment of MFPP, is not clear on the treatment length needed for successful relaxation of pelvic floor muscles but does state the need for frequent treatment (daily prior to biweekly spacing). Oyama et al. [12], who followed 21 women for 4.5 months after treatment, found that after physical therapy, pelvic tone increased over time, upwards to pretreatment baseline in the coccygeus muscles. Weiss documented episodic symptom flares in in the 52 patients who underwent Thiele massage, although the length of time from completion of treatment to the recurrence of pain was unspecified [13]. Jarvis et al. [4], who injected Botox into the puborectalis and pubococcygeus in 12 women with pelvic pain and hypertonicity, documented a mean reduction in pelvic floor manometry by 37% at week 4 but only by 25% by week 12.
The above studies indicate that although single-treatment modality with pelvic floor physical therapy or Botox can cause a reduction in pelvic tone and pain, the treatment response may be limited and transient. We theorize combined therapy can cause more long-lasting reductions in pelvic pain. It is our hypothesis that in combined treatment the pelvic trigger points are first reduced through the manual traction provided by physical therapy, and their reformation are prevented by blocking acetylcholine receptors as provided by Botox. Due to its retrospective nature, this study is not able to quantify the symptom-free interval attained through the use of combined therapy. However, there are some promising signs that the treatment may be long lasting. Specifically, in the 5 patients that underwent re-treatment, an average of 15 months (range, 5–30 months) passed before undergoing subsequent treatment.
A recently published study on complications with synthetic mesh (placed as a sling, vaginal, or abdominal mesh) found that at a mean follow-up of 2.4 years, 16% of patients experienced pelvic pain [14]. In our study, patients with a history of prior vaginal incontinence sling revision/removal, were significantly more likely to report an improvement in pain as compared to those that underwent removal of larger meshes (placed for the correction for prolapse). These results suggest that Botox combined with physical therapy may be an effective option for patients with chronic pelvic pain and a history of sling revision/removal. We are unsure why the group that included vaginal mesh for anterior/posterior repair did not report a significant improvement in pain. This observation may be a type II error correctable through increasing sample size.
In our case series, the patients that benefited the most from Botox and physical therapy were those that had trigger points on pelvic exam and generalized pelvic pain. Patients with another possible cause for their pelvic pain may not benefit as much from the treatment. In our case series, patients with a history of chronic bowel disorders were significantly more likely to report no improvement in pain. Chronic pelvic pain is multifactorial, and can be attributed to any or multiple organs in the pelvic region. Therefore, patients with chronic bowel disorders and MFPP should be counseled that they may continue to experience pelvic pain even with successful treatment of their trigger points. Patients with complex multifactorial chronic pelvic pain might benefit from multimodal treatment.
The number of complications associated with Botox and physical therapy were small and consisted of reversible conditions such as constipation, de novo transient urinary retention, and urinary tract infection. In our group, all of these conditions resolved spontaneously.
All of our procedures were performed under general anesthesia in order to make the procedure tolerable for patients. It is necessary for patients to be relaxed in order to provide enough manual traction on pelvic floor muscles in order to relieve trigger points. The office injection of Botox for the treatment of MFPP has been reported [15]. Although performing this procedure in the operating room increases costs, it allows us to perform a procedure that may be unbearable in the outpatient setting.
There are several limitations to this case series including retrospective design and small sample size. Of the 160 women who underwent treatment of MFPP with physical therapy and Botox at our institution, 110 did not meet our inclusion/exclusion criteria. The primary reason for exclusion of patients from the study was the occurrence of a concurrent procedure at the time of Botox with physical therapy. However, most of the current literature on Botox in pelvic pain is also limited by small sample size. Due to the retrospective design there was no standardized procedure for palpation of pelvic floor muscles and pelvic pain score. Likewise, a visual analog pain assessment tool would have standardized patient's pain perceptions. A major strength of our study is extended posttreatment follow-up that is much longer than any of the cited studies on either Botox or physical therapy.
In conclusion, Botox combined with soft tissue myofascial release physical therapy is a safe, new, innovative approach with minimal risk, for women with chronic pelvic pain related to MFPP. Furthermore, it may provide relief in women that have failed traditional pain treatment modalities. Prospective trials to investigate the true, long-term benefits of this treatment modality are needed in order to continue to offer it to women with chronic pelvic pain.
Figures and Tables
Table 1
Patient demographics (n=50)

Table 2
Comparing patient demographics, pain symptoms, past medical history, prior pelvic floor surgery between patients with reported improvement and no improvement in pain after treatment with combined Botox and physical therapy (n=50)
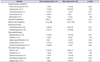
Values are presented as number (%), mean±standard deviation, or median (range).
a:In addition to generalized pelvic pain. b:Chronic bowel disease includes irritable bowel syndrome, inflammatory bowel disease, and diverticulosis. c:Vaginal mesh includes those used for anterior/posterior repair with/without placement of a concomitant incontinence sling.
ACKNOWLEDGMENTS
The findings have been presented at the 36th Annual American Urogynecologic Society Annual Scientific Meeting, October 13-17, 2015, Seattle, WA, USA (Poster #193).
References
1. Mathias SD, Kuppermann M, Liberman RF, Lipschutz RC, Steege JF. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996; 87:321–327.
2. Borg-Stein J, Simons DG. Focused review: myofascial pain. Arch Phys Med Rehabil. 2002; 83:3 Suppl 1. S40–S47. S48–S49.
3. Srinivasan AK, Kaye JD, Moldwin R. Myofascial dysfunction associated with chronic pelvic floor pain: management strategies. Curr Pain Headache Rep. 2007; 11:359–364.
4. Jarvis SK, Abbott JA, Lenart MB, Steensma A, Vancaillie TG. Pilot study of botulinum toxin type A in the treatment of chronic pelvic pain associated with spasm of the levator ani muscles. Aust N Z J Obstet Gynaecol. 2004; 44:46–50.
5. Abbott JA, Jarvis SK, Lyons SD, Thomson A, Vancaille TG. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006; 108:915–923.
6. Stata Corporation. Stata release 13: data analysis and statistical software. College Station (TX): StataCorp LP;2013.
7. Mense S, Simons DG, Russell IJ. Muscle pain: understanding its nature, diagnosis, and treatment. Philadelphia (PA): Lippincott Williams & Wilkins;2001.
8. Shah JP, Phillips TM, Danoff JV, Gerber LH. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol (1985). 2005; 99:1977–1984.
9. Kuan TS. Current studies on myofascial pain syndrome. Curr Pain Headache Rep. 2009; 13:365–369.
10. Lang AM. Botulinum toxin type A therapy in chronic pain disorders. Arch Phys Med Rehabil. 2003; 84:3 Suppl 1. S69–S73.
11. Raj PP. Botulinum toxin therapy in pain management. Anesthesiol Clin North America. 2003; 21:715–731.
12. Oyama IA, Rejba A, Lukban JC, Fletcher E, Kellogg-Spadt S, Holzberg AS, et al. Modified Thiele massage as therapeutic intervention for female patients with interstitial cystitis and high-tone pelvic floor dysfunction. Urology. 2004; 64:862–865.
13. Weiss JM. Pelvic floor myofascial trigger points: manual therapy for interstitial cystitis and the urgency-frequency syndrome. J Urol. 2001; 166:2226–2231.
14. Hansen BL, Dunn GE, Norton P, Hsu Y, Nygaard I. Long-term follow-up of treatment for synthetic mesh complications. Female Pelvic Med Reconstr Surg. 2014; 20:126–130.
15. Adelowo A, Hacker MR, Shapiro A, Modest AM, Elkadry E. Botulinum toxin type A (BOTOX) for refractory myofascial pelvic pain. Female Pelvic Med Reconstr Surg. 2013; 19:288–292.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download