Abstract
Purpose
We investigated the efficacy and safety of a readjustable midurethral sling (Remeex system) for stress urinary incontinence with female voiding dysfunction.
Materials and Methods
From May 2007 to December 2015, 151 women received the Remeex system. We excluded patients who presented with pelvic organ prolapse and/or who missed the regular follow-up. Finally, 102 patients were included in the study, and we divided them into 2 groups: group A (n=74), who did not present with female voiding dysfunction and group B (n=28), who presented with female voiding dysfunction. Female voiding dysfunction was defined as a maximal flow rate≤12 mL/s when the voided volume was ≥150 mL on preoperative uroflowmetry. We retrospectively reviewed the patients' medical records and compared surgical outcomes between groups A and B.
Results
There were no significant differences in subjective surgical outcomes and patient satisfaction between the 2 groups. Postoperative uroflowmetry showed that the maximal flow rate and voided volume decreased and the postvoid residual urine volume increased in both groups, and these changes were not significantly different between groups. The overall complication rate was 21.6% (22 of 102), and the complication rate was not significantly different between the 2 groups according to the modified Clavien-Dindo classification.
Stress urinary incontinence (SUI) is defined as a disease entity that presents with involuntary urine leakage during effort, exertion, or coughing [1]. SUI generates severe social, economic, and psychological problems that can have a significantly negative impact on women's health.
To treat SUI, several forms of retropubic colposuspension, pubovaginal slings, injectable bulking agents, and needle suspensions have been developed [2]. Recently, tension-free midurethral sling procedures have been used widely for SUI. Owing to the evolution of midurethral slings and their high efficacy and safety, retropubic midurethral slings and transobturator midurethral slings have been introduced into numerous urologic fields [3]. However, procedures using these 2 slings have a weakness: appropriate tension cannot be determined during the operation. Postoperative complications, such as acute urinary retention (AUR) or persistent urine leakage, can occur due to inappropriate tension [4]. The readjustable midurethral sling (Remeex system; Neomedic International, Terrassa, Spain) is an adjustable device that allows urologists to regulate midurethral tension intraoperatively and postoperatively, which theoretically can improve the success rate of the procedure and decrease the complication rate. Due to its adjustability, the Remeex system can be used for women with complicated SUI, those who have recurrent SUI after previous anti-incontinence operations, and those who have intrinsic sphincter deficiency (ISD), detrusor underactivity (DU), or underactive bladder (UAB).
The clinical diagnosis of female voiding dysfunction, such as DU or UAB, is dif f icult because it usually requires invasive pressure flow studies (PFSs), and its related symptoms and signs are relatively unknown [5]. Furthermore, few reports of the efficacy and safety of the Remeex system for SUI with female voiding dysfunction have been published. Therefore, we analyzed the surgical outcomes and perioperative complications for patients with SUI and female voiding dysfunction receiving the Remeex system.
This study was approved by the Ethics Committee of Kyungpook National University School of Medicine (approval number: KNUH 2016-04-007-001).
From March 2007 to December 2015, 151 patients underwent the Remeex system performed by a single surgeon at a single center. Candidates for the Remeex system were those who developed recurrent SUI, had mixed urinary incontinence, had ISD (Valsalva leak point pressure [VLPP] <60 cmH2O or maximal urethral closure pressure [MUCP] <20 cmH2O), had a low maximal flow rate (Qmax), or had a large postvoid residual (PVR) urine volume, which can be signs of DU. We excluded patients who presented with pelvic organ prolapse and/or who missed the regular followup. Finally, 102 patients were included in our study, and we divided them into 2 groups: group A (n=74), who did not present with female voiding dysfunction and group B (n=28), who presented with female voiding dysfunction. Female voiding dysfunction was defined as a Qmax ≤12 mL/s when the voided volume (VV) was ≥150 mL on preoperative uroflowmetry (UFM). We retrospectively reviewed the medical records of the patients and compared the clinical characteristics, surgical outcomes, and complication rates between groups A and B.
Before the Remeex procedure, all patients underwent a urodynamic study (UDS) consisting of UFM, filling cystometry, VLPP, MUCP profiles, and PVR urine volume. The preoperative evaluation included the past medical history, a physical examination of the pelvic organs, and routine laboratory blood and urine testing. In general, a PFS is not performed widely before sling operations, and definitions of DU and UAB are not yet well-established. Furthermore, DU is impossible to distinguish from bladder outlet obstruction (BOO) on the basis of symptoms, reduced flow rate, or increased PVR urine volume [6], so we defined “female voiding dysfunction” as a Qmax≤12 mL/s when the VV was ≥150 mL on preoperative UFM. Additionally, ISD was defined as a VLPP<60 cmH2O or an MUCP<20 cmH2O when the bladder was filled with >150 mL, as it is usually defined in urologic fields.
All patients were placed in the dorsal lithotomy position. A urethral catheter was inserted, and the bladder was emptied. Hydrodissection of the anterior vaginal wall was performed first. The anterior vaginal wall was incised about 2.5 cm on the midurethra and dissected from periurethral tissues. A transverse abdominal incision was made about 3 cm above the symphysis pubis until the rectus fascia was exposed. Both introducers were passed laterally from the dissected periurethral tissues into the Retzius space, penetrating the rectus fascia. A cystourethroscopic examination was performed to confirm that there was not any urethral or bladder injury. The threads connected with mesh were passed via needle holes into the introducers and pulled up until they approached the abdominal incision. A varitensor was placed above the rectus fascia and both threads were passed through the varitensor, fixed, and cut. The sling mesh was positioned at the midurethra by placing a metzenbaum between the mesh and midurethra for tension-free placement, and the manipulator was rotated clockwise until the varitensor approached about 4 cm above the rectus fascia. (In our center, varitensors are positioned approximately 4 cm above the rectus fascia, not 3 cm above it, so as not to induce postoperative AUR). All incisions were closed in the usual manner, leaving the manipulator seen above the abdominal incision. One day after operation, the urethral catheter was removed and the patient was asked to wait until he or she felt the need to urinate spontaneously. Then, the patient was asked to stand up, perform Valsalva maneuvers, and undergo UFM. If any urine leakage was detected, the manipulator was adjusted clockwise. On the other hand, if the PVR urine volume was ≥100 mL, the manipulator was turned counterclockwise to reduce the tension on the sling. If the PVR urine volume was <100 mL, urine leakage decreased and the patient was satisfied with operative outcomes. The above-mentioned procedure was performed once more, and then the manipulator was removed from the varitensor. Finally, the varitensor was implanted between subcutaneous tissue and the rectus fascia, and the removed manipulator was given to the patient for any sling readjustment that might be needed during regular follow-up.
The postoperative follow-up was scheduled at 2 weeks; 2, 6, and 12 months; and annually after discharge. During regular follow-up, patients underwent a stress test and UFM. Additionally, patients were asked to answer several questions related to subjective cure and satisfaction. Definitions for the subjective patient surgical outcomes were as follows: “cure” was defined if urinary incontinence no longer occurred at all; “improvement” in urinary incontinence was quite rare and unnoticeable; and all the other outcomes were defined as “failure.” Patients were also asked to evaluate their satisfaction with surgical outcomes by selecting 1 of 5 grades: very satisfied, satisfied, neither satisfied nor dissatisfied, dissatisfied, very dissatisfied. Subjective surgical outcomes and patients' satisfaction were measured 6 months after the Remeex operation.
All complications occurring perioperatively were analyzed according to the modif ied Clavien-Dindo classification.
Differences in the mean values of age, parity, duration of hospital stay, follow-up periods, Qmax, VV, and PVR urine volume were analyzed between groups using the Student t-test. The chi-square test and Fisher exact test were used to compare subjective surgical outcomes, patient satisfaction, and the complication rate. Statistical analysis was performed using SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA), and p<0.05 was considered statistically significant.
Seventy-four patients (group A, 72.5%) did not present with female voiding dysfunction, while 28 patients (group B, 27.5%) did. All patients in group B were treated with an α-blocker more than 3 months before surgery, but their Qmax did not increase to >12 mL/s. Patients' characteristics are shown in Table 1. There was one patient with a neurogenic bladder caused by myasthenia gravis in group B who showed low ISD, low MUCP, and a large PVR urine volume.
Preoperative UFM showed that the Qmax values in groups A and B were 22.7±5.9 and 9.2±2.5 mL/s, respectively (p<0.001) (Table 2). VV values in groups A and B were 259.6±69.4 and 221.7±56.6 mL, respectively (p=0.011). PVR urine volumes in groups A and B were 16.2±23.9 and 29.0±35.9 mL, respectively, but these were not significantly different (p=0.125). The 2-month postoperative UFM showed that the Qmax and VV decreased and PVR urine volume increased slightly, and these variations were not significantly different between the 2 groups (Table 3). Other results of the preoperative UDS are shown in Table 2. There was 1 patient whose UDS finding showed DO in group B, which could be a sign of detrusor hyperactivity with impaired contractile function. Only compliance was significantly different between the 2 groups (p=0.043).
With respect to subjective surgical outcomes, 61 patients (59.8%) described themselves as “cured,” 37 (36.3%) as “improved,” and 4 (3.9%) reported “failure.” Twenty patients (19.6%) were “very satisfied”, 54 (52.9%) were “satisfied,” 24 (23.5%) were “neither satisfied nor dissatisfied,” 3 (2.9%) were “dissatisfied,” and 1 (1.0%) was “very dissatisfied.” There were no significant differences in subjective surgical outcomes and patient satisfaction between the 2 groups (Table 4). The mean number of tension regulations performed on postoperative day 1 was 1.4±0.8 in group A and 3.0±1.5 in group B; thus, it was significantly higher in group B (p<0.001).
The overall complication rate was 21.6% (22 of 102). There were no significant differences in complication rates between the 2 groups according to the modified Clavien-Dindo classification (Table 5). Eight patients experienced de novo urgency, which was the most common complication and usually required anticholinergic therapy. All 8 patients who experienced de novo urgency showed decreased Qmax after operation, but the decrease was not statistically significant (preoperative Qmax, 20.1±9.7 mL/s vs. postoperative Qmax, 14.8±5.5 mL/s, p<0.199). Immediate (i.e., on postoperative day 1) AUR was observed in 3 patients. These 3 patients had a Foley catheter reinserted, and the manipulator was adjusted counterclockwise. Recurrent SUI was observed in 3 patients, and they underwent the sling readjustment procedure. All recurrent SUI cases appeared 12 months after the initial Remeex operation, and the readjustment procedure was performed 3 months after the appearance of the recurrent SUI to rule out other causes of SUI, such as urinary infection. Three patients experienced wound infection, and one of these underwent sling removal.
Conservative, medical, and surgical management have historically been used to treat SUI. Surgical management was the most definite treatment for SUI, and tension-free vaginal tape (TVT) was first introduced by Ulmsten et al. [7] in 1996. TVT, which is a representative type of sling, is based on the Integral theory [8] that the midurethra plays a significant role in urinary continence. In 2004, Delorme et al. [9] reported that transobturator tape (TOT), which uses an obturator foramen to pass the tape, is more convenient and efficient than is TVT and shows similar operative outcomes and complication rates. De Leval [10] further improved the TOT method and developed the inside-out TVT obturator, and it became the new way of performing the sling operation. To date, numerous surgical techniques that can allow tension adjustment to reduce complications, such as urinary leakage or retention, have been introduced [11]. These include the Remeex system, transobturator adjustable tape, and adjustable transvaginal tape.
According to a report by Mazouni et al. [12], 60% of SUI patients had voiding difficulty after TVT surgery. The persistence of voiding difficulty or incontinence is a frequent problem after the midurethral sling procedure due to how tightly or loosely the mesh is positioned [13]. The weaknesses of sling surgery are the excess tension of the sling and postoperative BOO, and new methods for determining appropriate sling tension have been developed as a result [14]. The other weaknesses are the persistence of urine leakage. If SUI reappears, a new surgical procedure should be performed to correct the incontinence, but it is very challenging due to tissue adhesion or remnant mesh. However, the Remeex system can overcome these weaknesses of the sling operation for SUI. The Remeex system not only allows the surgeon to position the sling with adequate tension during surgery but also allows the surgeon to loosen or tighten the sling postoperatively to achieve continence as well as to maintain adequate voiding function. Furthermore, as described in Table 2, patients in group B showed significantly lower bladder compliance than did patients in group A. In 2015, Liao et al. [15] analyzed 1,490 women undergoing videourodynamic studies, and they showed that low bladder compliance is associated with decreased VV and Qmax and increased PVR urine volume. Thus, patients with low bladder compliance could be good candidates for the Remeex operation.
Theoretically, the Remeex system could be used for patients with voiding difficulty, such as DU. With respect to DU, a recent study concluded that much about DU remains ambiguous and recognized the limitations of the current definition. The International Continence Society [16] defines DU as “a contraction of reduced strength and/or duration, resulting in prolonged bladder emptying and/or a failure to achieve complete bladder emptying within a normal time span.” However, this definition does not concretely define prolonged bladder emptying or a normal time span. Numerous methods have been evaluated to determine the strength of contraction, but none can calculate the contraction duration, a key factor in the definition. Furthermore, although DU is thought to be a common geriatric condition, DU has not received much scientific attention, primarily because the clinical characteristics of DU have not been clearly defined and understood and a gold-standard diagnosis for DU has not yet been established from a urodynamic standpoint [17].
Despite a lack of definite knowledge about DU, elderly women show a prevalence of DU ranging from 12% to 45%. Other studies have reported that 45% of older women who undergo evaluation for lower urinary tract symptoms (LUTS) show evidence of DU [18]. However, although DU is highly prevalent, DU is largely under-researched compared to other lower urinary tract dysfunctions, such as BOO or DO. Moreover, there is no effective and simple treatment [19]. Thus, urologists should bear in mind these vague characteristics regarding epidemiology and diagnostic approaches when treating patients with DU.
Choi et al. [20] defined female voiding dysfunctions as a Qmax of ≤15 mL/s, BOO as a Qmax of ≤15 mL/s and detrusor pressure of >20 cmH2O at Qmax, and DU as a Qmax<5 mL/s with detrusor pressure<20 cmH2O at Qmax on PFS. However, at our center, as at other urologic centers, PFS is not performed routinely before sling operations. In general, preoperative UDS is used to confirm SUI and check VLPP, bladder compliance, MUCP, and functional bladder capacity, so preoperative PFS is performed before a sling operation only in special cases, such as neurogenic cystopathy. Therefore, our study has a limitation in that calculation of detrusor pressure at Qmax was impossible. Accordingly, we did not use specific terminology such as DU, BOO, or UAB and instead defined “female voiding dysfunction” as described above. Furthermore, the mean PVR urine volume in group B was larger than that in group A, but not statistically significantly so; thus, we could not define female voiding dysfunction using both low Qmax and high PVR urine volume. We think that this is because of the small number of patients, and we will analyze data from a larger number of patients via a multicenter study in the future to overcome this weakness. Another limitation is that most patients did not undergo both a pre- and postoperative 1-hour pad test, so an analysis of objective surgical outcomes was impossible.
In contrast to LUTS, such as DO, few studies have focused on the cause and treatment of DU [21]. It is thought that the Remeex system could allow surgeons to regulate midurethral tension, either intra- or postoperatively, but there have been few studies of the efficacy and safety of the Remeex system in SUI patients with female voiding dysfunction. Thus, to our knowledge, results of our study could uniquely guide the surgeon to understand and treat SUI patients with female voiding dysfunction adequately.
The Remeex system allows surgeons to regulate the patient's urethral sling tension with a low complication rate, not only for an immediate adjustment but also for a delayed readjustment in cases of treatment failure. Thus, the Remeex system is thought to be more efficacious than the TVT or TOT systems. In particular, female patients who not only present with SUI but also present with voiding dysfunction, which could be signs of DU, could be good candidates for the Remeex system.
Figures and Tables
Table 1
Clinical characteristics of the patients

Table 2
Pre- and postoperative uroflowmetry and preoperative urodynamic study findings

Table 3
Comparison of variations in Qmax, VV, and PVR urine volume between groups A and B

| Variation | Total | Group A | Group B | p-value |
|---|---|---|---|---|
| Qmax (mL/s) | −1.3±6.3 | −1.65±7.29 | −0.36±1.93 | 0.165 |
| VV (mL) | −17.0±74.6 | −18.62±76.19 | −12.86±71.21 | 0.729 |
| PVR (mL) | 6.7±38.4 | 6.81±32.94 | 6.46±50.91 | 0.968 |
Table 4
Surgical outcomes
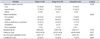
Table 5
Complications according to the modified Clavien-Dindo classification

References
1. Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002; 21:167–178.
2. Dmochowski RR, Blaivas JM, Gormley EA, Juma S, Karram MM, Lightner DJ, et al. Update of AUA guideline on the surgical management of female stress urinary incontinence. J Urol. 2010; 183:1906–1914.
3. Seo MY, Noh JH. Long-term outcome of the readjustable sling procedure for female stress urinary incontinence with intrinsic sphincter deficiency or recurrence. Korean J Urol. 2014; 55:124–128.
4. Park BH, Kim JC, Kim HW, Kim YH, Choi JB, Lee DH. Midterm efficacy and complications of readjustable midurethral sling (Remeex system) in female stress urinary incontinence with recurrence or intrinsic sphincter deficiency. Urology. 2015; 85:79–84.
5. Gammie A, Kaper M, Dorrepaal C, Kos T, Abrams P. Signs and symptoms of detrusor underactivity: an analysis of clinical presentation and urodynamic tests from a large group of patients undergoing pressure flow studies. Eur Urol. 2016; 69:361–369.
6. Osman NI, Chapple CR, Abrams P, Dmochowski R, Haab F, Nitti V, et al. Detrusor underactivity and the underactive bladder: a new clinical entity? A review of current terminology, definitions, epidemiology, aetiology, and diagnosis. Eur Urol. 2014; 65:389–398.
7. Ulmsten U, Henriksson L, Johnson P, Varhos G. An ambulatory surgical procedure under local anesthesia for treatment of female urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1996; 7:81–85.
8. Petros PE, Ulmsten UI. An integral theory and its method for the diagnosis and management of female urinary incontinence. Scand J Urol Nephrol Suppl. 1993; 153:1–93.
9. Delorme E, Droupy S, de Tayrac R, Delmas V. Transobturator tape (Uratape): a new minimally-invasive procedure to treat female urinary incontinence. Eur Urol. 2004; 45:203–207.
10. de Leval J. Novel surgical technique for the treatment of female stress urinary incontinence: transobturator vaginal tape inside-out. Eur Urol. 2003; 44:724–730.
11. Jo DG, Yang SA, Seo JT. Effects of transobturator adjustable tape sling procedure on the therapeutic outcome in patients with stress urinary incontinence and detrusor underactivity. Int Neurourol J. 2010; 14:20–25.
12. Mazouni C, Karsenty G, Bretelle F, Bladou F, Gamerre M, Serment G. Urinary complications and sexual function after the tension-free vaginal tape procedure. Acta Obstet Gynecol Scand. 2004; 83:955–961.
13. Nitti VW, Carlson KV, Blaivas JG, Dmochowski RR. Early results of pubovaginal sling lysis by midline sling incision. Urology. 2002; 59:47–51.
14. Yoo DH, Noh JH. Readjustable sling procedure for the treatment of female stress urinary incontinence with intrinsic sphincter deficiency: preliminary report. Korean J Urol. 2010; 51:420–425.
15. Liao JY, Lin YH, Liang CC, Hsieh WC, Lee SJ, Tseng LH. Monitoring bladder compliance using end filling detrusor pressure: Clinical results and related factors. Taiwan J Obstet Gynecol. 2015; 54:709–715.
16. Taylor JA 3rd, Kuchel GA. Detrusor underactivity: clinical features and pathogenesis of an underdiagnosed geriatric condition. J Am Geriatr Soc. 2006; 54:1920–1932.
17. Jeong SJ, Kim HJ, Lee YJ, Lee JK, Lee BK, Choo YM, et al. Prevalence and clinical features of detrusor underactivity among elderly with lower urinary tract symptoms: a comparison between men and women. Korean J Urol. 2012; 53:342–348.
18. Abarbanel J, Marcus EL. Impaired detrusor contractility in community-dwelling elderly presenting with lower urinary tract symptoms. Urology. 2007; 69:436–440.
19. Chapple CR, Osman NI, Birder L, van Koeveringe GA, Oelke M, Nitti VW, et al. The underactive bladder: a new clinical concept? Eur Urol. 2015; 68:351–353.
20. Choi YS, Kim JC, Lee KS, Seo JT, Kim HJ, Yoo TK, et al. Analysis of female voiding dysfunction: a prospective, multi-center study. Int Urol Nephrol. 2013; 45:989–994.
21. Jhang JF, Jiang YH, Lee CL, Kuo HC. Long-term follow up and predictive factors for successful outcome of transurethral incision of the bladder neck in women with detrusor underactivity. J Formos Med Assoc. 2016; 115:807–813.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download