Abstract
Purpose
Estrogens act through interaction with 2 receptor subtypes, ER alpha (ERα) and ER beta (ERβ), in human prostate. The aim of the present study was to semiquantitatively assess the differential expression of ER subtypes in human benign prostatic hyperplasia (BPH) by use of immunocytochemistry (IHC) methods and to explore their relationship with various measures of BPH.
Materials and Methods
A total of 45 patients with BPH undergoing transurethral resection of the prostate and 22 patients with bladder cancer with normal prostate undergoing surveillance cystoscopy were studied as cases and controls, respectively. Quantitative immunolabeling of ER subtypes was scored by use of a semiquantitative scale. Also, correlations were assessed between ER levels in prostate and various measures of BPH.
Results
Overall, we found strong immunostaining for ERα in stroma and for ERβ in epithelium, respectively. The IHC score for ERα differed significantly between BPH patients and controls in both stroma (p≤0.001) and epithelium (p=0.008), respectively. The ERβ IHC score was also significantly higher in the epithelium of BPH patients (p=0.01). Also, we found a significant correlation between prostatic ER levels and various clinical measures of BPH.
The exact etiology of benign prostatic hyperplasia (BPH) is still not fully elucidated. It is known, however, that complex epithelial-stromal interactions in the setting of a fitting hormonal milieu are responsible for BPH development [1]. Estrogens have long been suspected to play an important role in prostate growth, but their role is incompletely understood [23]. Estrogenic action is mediated by specific intracellular estrogen receptors (ERs) and their activation, which can occur independent of the serum estrogen level [4]. Two subtypes of ER have been identified, ER alpha (ERα) and ER beta (ERβ), in human prostate [56]. ERα is expressed primarily in prostatic stromal cells and ERβ expression is chiefly localized to prostatic epithelium [7891011121314]. However, the localization of these receptors is not exclusive and remains contentious. Many studies have demonstrated increased ER expression and differential expression of receptor subtypes in BPH specimens as well as cell cultures [810151617].
Recent work with knockout mice models has been invaluable in understanding the specific roles of ERα and ERβ in prostate growth and differentiation [418192021]. The current evidence suggests a proliferative role for ERα and an antiproliferative role for ERβ. Royuela et al. [10] investigated the differential expression of ERα and ERβ by using immunohistochemistry (IHC) methods and assessed ER levels in normal, hyperplastic, and carcinomatous prostatic tissue. However, quantitative data are still sparse and lacking regarding the expression of ERs, and the relationship between receptor levels and measures of BPH and lower urinary tract symptoms (LUTS) have not been explored previously.
We hypothesized that ER levels in prostatic tissue should correlate with the various measures of BPH and LUTS. We used the Ki-67 index assay to objectively quantify cell proliferation. The aim of the present study was to semiquantitatively assess the differential expression of ER subtypes in prostatic tissue in men with BPH by use of IHC methods and to explore their relationship with various clinical measures of BPH.
This case-control study was conducted in our department after receiving clearance from SMS Medical College, Jaipur ethics committee (IRB No. 1379/MC/EC/2015). A total of 45 patients with a diagnosis of clinical BPH with bothersome LUTS (moderate to severe International Prostate Symptom Score [IPSS]) undergoing transurethral resection of the prostate (TURP) were included in the study (group A). Informed consent was received from all patients. Exclusion criteria were endocrine disorders, recent or long-term use of any hormonal agents causing androgen manipulation (e.g., dutasteride), significant comorbidities like stroke or neurogenic bladder affecting LUTS, previous lower urinary tract surgery, active urinary tract infections, current indwelling catheter or history of urinary retention within 1 month of inclusion, diagnosis of prostate or bladder cancer, vesical calculus, urethral stricture, and prostate-specific antigen (PSA)>4 ng/dL. Control samples were histologically normal prostatic tissue obtained from 22 patients with bladder cancer without evidence of bladder outlet obstruction who underwent surveillance cystoscopy (group B).
Preoperatively, the patients' medical history and physical examination results, including digital rectal examination and urinalysis, were obtained. Also, BPH-related parameters such as serum PSA, prostate volume, maximal flow rate (Qmax), IPSS, and postvoid residual urine volume (PVR) were recorded. Serum hormonal assays of total testosterone and estradiol were measured in an early morning blood sample. All patients underwent standard TURP. The prostatic tissue chips were sent in 10% formalin for histopathological examination IHC. ER distribution and quantification and Ki-67 were assessed by use of IHC techniques as described below.
All formalin-fixed prostatic tissues were embedded in paraffin and 5 samples were randomly selected from each prostate. A 5-µm section was prepared from each sample. Sections were further processed by heating at 60℃–70℃ in an oven for 30 to 40 minutes and dewaxing with xylene and alcohol for 2 cycles of 10-minute duration each. The slides were then deionized in distilled water for 5 minutes and washed in peroxidase solution for 10 to 15 minutes, after which Tris buffer was applied for 5 minutes. Microwave antigen retrieval was carried out by placing the slides in citrate buffer (Retrieval Box; citrate buffer antigen retrieval protocol) in a Decloaking Chamber (Biocare Medical Inc., Concord, CA, USA) at 125℃. After adequate decloaking and cooling, slides were rinsed with Tris buffer and sections were marked. A total of 3 to 4 drops of Background Sniper (Biocare Medical Inc.) was applied for 15 minutes. The slides were then again washed with Tris buffer.
Primary antibodies were applied for 1 hour and subsequently rinsed with Tris buffer. After application of polymer for 30 minutes, slides were again rinsed with Tris buffer. Diaminobenzidine was applied for 5 minutes and the samples hydrated. After applying hematoxylin for 2 minutes, slides were rinsed and dehydrated with alcohol and xylene for 2 cycles of 10-minute duration and then mounted.
Negative and positive controls were obtained to check the specificity of the IHC procedure. Hypothalamus tissue sections for ERβ and breast cancer tissue for ERα were taken as positive controls (Fig. 1A, B). For Ki-67, tonsil tissues were taken as positive controls.
The following antibodies were used: ERα mouse monoclonal antibody in 1:200 dilution (Acris Antibodies Inc., San Diego, CA, USA), ERβ monoclonal antibody in 1:50 dilution (Acris Antibodies Inc.), and pretitrated mouse monoclonal anti-Ki-67 antibodies (clone MB67, Diagnostic BioSystems, Pleasanton, CA, USA).
Quantitative immunolabeling of ER subtypes was scored by use of a semiquantitative scale incorporating both the proportion of positively stained target cells (scored on scale of 0–5) and the staining intensity (scored on scale of 0–3), similar to the Allred score used in breast cancer [22]. The proportion score (PS) was given as 0 (none), 1 (1%), 2 (1%–10%), 3 (10%–33%), 4 (33%–66%), and 5 (>66%). The intensity score (IS) was given as 0 (no staining), 1 (weak staining), 2 (intermediate), and 3 (strong). A sum of PS and IS score of 2 or less was considered negative and all other scores were considered positive. Under 40× magnification, the area with highest intensity was identified. Under 400×, 100 cells were counted per field and the percentage of ER was calculated as the number of positively stained cells per 100 cells counted and an IS also calculated. The total score from 5 sections was summed and a mean score was calculated. Independent scores for ERα in epithelium (EERα) and stroma (SERα) and ERβ in epithelium (EERβ) and stroma (SERβ) were measured.
Similarly, for the Ki-67 assay, after tissue processing, immunoquantification was performed by calculating the percentage of positively stained cells. Each slide was scanned at 40× magnification to locate areas with maximum positive cells. Then at 400× magnification, 1,000 cells were counted, 500 cells from stroma and 500 cells from epithelium, and the Ki-67 index was calculated by counting 500 to 1,000 random nuclei under the light microscope at 400× magnification.
Continuous data were analyzed by using 2-tailed t-test, and Pearson correlation coefficient was used to correlate the various measures of BPH and LUTS with quantitative measures of ERα and ERβ in the prostatic stroma and epithelium. A p-value of <0.05 was considered statistically significant.
Comparison of baseline parameters between BPH patients and controls is shown in Table 1. The mean (range) age of the patients was 66.1 years (45–80 years) and 64.2 years (55–75 years) in groups A and B, respectively. The serum hormonal profile was similar in both groups (p>0.05). The mean serum total testosterone was 351 and 395 ng/dL in groups A and B, respectively, and serum estradiol was 28 and 22.4 pg/dL in groups A and B, respectively. There was a significant difference in mean prostate size, PVR, IPSS, and Qmax between groups A and B (p<0.01). There was no statistically significant difference in mean PSA between group A (2.05 ng/dL) and B (1.8 ng/dL) (p=0.10).
IHC evaluation of prostatic specimens by use of monoclonal antibody to ER subtypes demonstrated variable staining patterns in both stroma and epithelium as well as between BPH patients and controls (Fig. 2A–G). In group A, positive immunostaining (defined as IHC score>2) for ERα was found in stromal nuclei from 30 prostates (66.7%) and in epithelial nuclei from 27 prostates (60%), whereas in group B, only 4 patients each (18.2%) showed positive immunostaining in stroma and epithelium. Also, positive immunoreactivity for ERβ was observed in epithelial nuclei from 31 prostates (80%) and stromal nuclei from 5 prostates (11%) in BPH patients. In controls, ERβ expression was observed in epithelial nuclei of 12 patients (54.5%) and stromal cells in only 2 patients (9%). Thus, the expression of ER was heterogeneous in both BPH patients and normal controls. Overall, we found that immunostaining was stronger for ERα in stroma and ERβ in the epithelium and the immunostaining was confined mostly to the basal epithelial cells for both ER subtypes. Additionally, we also observed that positively stained cells in both stroma and epithelium tended to cluster together and lie in close proximity (Fig. 2E).
Semiquantitative IHC scoring was carried out as described previously for both ER subtypes in both stroma and epithelium (Fig. 3). There was a significant difference in IHC score for ERα between BPH patients and controls in both stroma (p≤0.001) and epithelium (p=0.008), respectively. The ERβ IHC score also was significantly higher in the epithelium of BPH patients (p=0.01). However, we could not find any significant difference in stromal ERβ IHC score between the 2 groups (p=0.28).
All BPH patients showed positive immunostaining for Ki-67, whereas only 14 patients (63.6%) in the control group were positive. The Ki-67 index was significantly higher in BPH patients than in controls (0.46±0.42 vs. 1.23±1.0, p=0.02).
Among the various clinical parameters, prostate size correlated significantly with PSA (p=0.001) and IPSS (p=0.003). PSA also correlated significantly with serum estradiol (p=0.02). Likewise, there was significant correlation between IPSS and Qmax (p=0.01) (Table 2).
There was a significant positive correlation between SERα and age (r=0.37, p=0.013), PSA (r=0.320, p=0.03), prostate size (r=0.51, p=0.001), and IPSS (r=0.32, p=0.03). EERα positively correlated with serum estradiol (r=0.53, p=0.001), serum estradiol/total testosterone ratio (r=0.44, p=0.003), prostate size (r=0.38, p=0.01), and IPSS (r=0.29, p=0.04). EERβ correlated significantly with serum estradiol only (r=0.56, p=0.001). There was a positive correlation between EERβ and serum testosterone but it was not statistically significant (r=0.262, p=0.08). No significant correlation was seen with SERβ. Both EERα and EERβ showed good positive correlation (r=0.25); however, again this was statistically insignificant (p=0.09) (Fig. 4). These results clearly indicated that ERs (especially SERα) are associated with various clinical measures of LUTS (like IPSS).
Localization of ERs in the prostate has been a matter of intense debate as conflicting results have been reported by various researchers. Most animal studies have reported that ERα is primarily localized to the stromal compartment and that ERβ is expressed mainly in the prostatic epithelium [78910111415161923]. Probable explanations for the variations in results include procedural differences such as the type of tissue studied or the antibodies used [3]. Few studies to date have assessed ER distribution in BPH patients and normal prostate [101617]. To understand the role of ERs in prostate biology, it is imperative to study their distribution in human prostate [7].
We are probably the first to use an IHC-based semi-quantitative assay to objectively assess the ER content in the prostate. We found that ERα was distributed in both stromal and epithelial compartments in a significant number of BPH patients (67% and 60% in stroma and epithelium, respectively). In contrast to older studies and similar to a few newer studies, we found ERα expression in some basal epithelial as well as stromal cells [81524]. ERβ positivity was seen in 78% of BPH patients in epithelium but was minimally expressed (11%) in the stromal compartment. Royuela et al. [10] found that 30% of patients with BPH had ERβ positivity in epithelium. In contrast, few authors have reported ERβ expression in stromal cells [72425]. Compared with the expression in normal controls, expression of both ER subtypes was significantly higher in BPH patients. This differential expression of ERs in both stromal and epithelial compartments may be related to the different role of ERs in prostate growth.
The Ki-67 index was significantly higher in BPH patients than in controls. When we compared ER expression in BPH patients and controls, both receptor subtypes were found to be significantly overexpressed in BPH patients with the exception of stromal ERβ. The IHC score for ERα was significantly higher for BPH patients in both stroma and epithelium. The ERβ IHC score also was significantly higher in epithelium of BPH patients. Thus, up-regulation of these receptors provides indirect objective evidence for their possible role in prostatic hyperplasia.
Many studies support the concept that SERα is responsible for estrogen-mediated actions in target epithelial cells [2627]. Increased accumulation of estradiol in stromal cells obtained from BPH specimens also suggests a possible role of ERα in BPH [28]. Others found no role of ERα in normal development of the prostate [19]. Studies in estrogen receptor knockout (ERKO) mice have demonstrated that SERα is responsible for estradiol-induced squamous metaplasia in adult prostates [27]. Similarly, studies using α ERKO and β ERKO mice have shown that stromal hyperplasia, epithelial PIN lesions, and inflammatory cell infiltration are mediated through stromal ERα [19]. In our study, in addition to their up-regulation, we also found that SERα showed significant correlations with various measures of BPH like PSA, prostate size, and IPSS. The close association of prostate size and PSA with SERα thus provides a hint to its role in mediating stromo-glandular proliferation, which is a hallmark of BPH. We could not explain the positive relationship between SERα and PSA, as PSA is an androgen-driven gene product. Probably, certain unknown ER-mediated mechanisms do exist that are responsible for up-regulation of AR-controlled genes and products. Although there was no correlation between SERα and the serum estradiol level in our study, ERs can act peripherally independent of serum estradiol levels [4]. Recently, Shapiro et al. [25] also reported localization of ERα in both stroma and epithelium in the fetal prostate. Further complicating the role of ERs in BPH, Risbridger et al. [27] using tissue recombination demonstrated that both epithelial and stromal ERα are required to induce a complete estrogenic response in the prostatic epithelium. More recently, Nicholson et al. [21] using ERKO mice demonstrated that ERα was the key mediator of bladder complications of BPH and that these complications can be prevented by using selective estrogen receptor modulators. We also found very strong associations between EERα levels and various measures of BPH, such as prostate size and IPSS. Notably, significant correlations between serum estradiol and the serum estradiol/total testosterone ratio and EERα levels were also found in our study. We commonly observed a close spatial proximity between ER-positive stromal and epithelial cells, which is an ideal setting for a paracrine mode of communication between both types of cells. Thus, our results do provide some indirect evidence for the important role of ERα in the pathogenesis of BPH and the existence of a complex interplay between epithelial and stromal components. Therefore, we believe that estrogenic action may be primarily mediated by SERα in the pathogenesis of human BPH.
Our findings showed that ERβ immunoreactivity was highest in epithelium and minimally observed in the stroma. Although we found a significant correlation between EERβ and serum estradiol, meaningful associations with other measures of BPH could not be derived. Although the available literature suggests ERβ to be the predominant receptor type in prostate, their role remains ill-defined [24]. However, studies using β ERKO mice have provided indirect evidence for the role of ERs in prostate growth. Dupont et al. [20] reported that ERβ probably has a role in normal growth and differentiation of the prostate because no prostate phenotype was observed in β ERKO mice. Similarly, Imamov et al. [29] showed that β ERKO mice had less differentiated prostate. In contrast Krege et al. [30] demonstrated that β ERKO mice do develop prostatic hyperplasia with aging, suggesting a negative role for ERβ in the regulation of glandular epithelial cell proliferation. The role of ERβ in stromal proliferation has shown contrasting results with β ERKO mice and remains inconclusive [1820]. In our study, the findings like significant ERβ immunoreactivity in epithelium and its relationship with serum estradiol do suggest that certain unknown mechanisms might be involved in the pathogenesis of BPH, which needs further analysis. Thus, the role of ERβ still merits further analysis and scrutiny for a better understanding of the pathogenesis of BPH.
The presence of multiple isoforms of ERβ further complicates the investigation of this receptor as these ER isoform variants can act as constitutive activators, enhancers, or negative regulators of estrogenic actions. In addition, IHC assays may vary in their sensitivity, technique of antigen retrieval, and ability to detect unknown isoforms [23]. Our study findings demonstrate the differential expression of ERα and ERβ in epithelial and stromal tissues in prostate. Importantly, up-regulation of ERα in BPH patients as well as significant correlation between various measures of BPH and ERα levels in the prostate lends support to the hypothesis that SERα may be the key mediator of estrogenmediated prostatic hyperplasia. Also, EERα and SERα might be complementary in their actions, probably through paracrine effects of estrogens on the prostate epithelium. The role of ERβ in BPH remains controversial and we could not conclusively arrive at any opinion. However, our findings do provide some evidence supporting the notion that estrogen may play a crucial role in the pathogenesis of BPH. Also, this difference in compartmental expression, as well as differential binding of the two ERs for various ligands, enhancers, and coactivators, might explain the differential action of estrogens on the prostate gland and can be targeted for treatment of various prostate diseases in the near future.
However, there were certain shortcomings to our study. First, this was a cross-sectional study and thus the dynamic correlation between the measures of LUTS and estrogen receptors cannot be derived from our study. Second, our study was based on IHC only and thus far-reaching conclusions should not be derived solely based on these results.
Figures and Tables
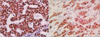 | Fig. 1Positive controls for estrogen receptors (ERs). Breast cancer tissue for ER alpha (A) and hypothalamus tissue sections for ER beta (B) were taken as positive controls (H&E, ×40). |
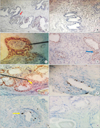 | Fig. 2Immunohistochemical analysis of estrogen receptor (ER) subtypes and Ki-67 expression in prostatic specimens from benign prostatic hyperplasia (BPH) patients (A, C, E, G) and controls (B, D, F, H). (A) Section from BPH specimens shows positive immunostaining (red arrow) for ER beta (ERβ) (H&E, ×40). (B) Representative area from normal prostate shows absence of ERβ immunostaining (H&E, ×40). (C) Intense ERβ positivity was primarily localized to epithelial nuclei (black pointer) in BPH tissues, whereas (D) nuclear staining was sparse (blue arrow) and seen mostly in epithelium of normal prostates (C, D: H&E, ×400). (E) In BPH specimens, immunoreactivity for ER alpha (ERα) was intensely expressed in both epithelial and stromal compartments. Note the close proximity between immunoreactive epithelial and stromal cells (black pointer and yellow arrow) (H&E, ×400). (F) ERα was expressed with much lower intensity in both stromal and epithelial compartments in controls (H&E, ×400). (G) Abundant nuclear staining for Ki-67 (yellow arrow) was found in BPH specimens, whereas (H) a representative area from normal prostate shows absent staining (G, H: H&E, ×400). |
 | Fig. 3Comparison of immunocytochemistry (IHC) scores of both estrogen receptor (ER) subtypes in benign prostatic hyperplasia (BPH) patients vs. normal controls. *Significant difference (p<0.05). |
 | Fig. 4Significant correlation between various measures of benign prostatic hyperplasia (BPH) and estrogen receptor (ER) in prostatic stroma and epithelium. (A) ER alpha (ERα) in stroma vs. age (r=0.37). (B) ERα in stroma vs. prostate-specific antigen (PSA) (r=0.32). (C) ERα in epithelium vs. International Prostate Symptom Score (IPSS) (r=0.32). (D) ERα in epithelium vs. prostate size (r=0.38). (E) ERα in stroma vs. prostate size (r=0.51). (F) ERα in epithelium vs. serum estradiol (r=0.53). (G) ER beta (ERβ) in epithelium vs. serum estradiol (r=0.56). A p-value of <0.05 was considered statistically significant. |
Table 1
Comparison of baseline parameters between control and BPH patients

Table 2
Pearson correlation coefficient between ER and various measures of LUTS

ER, estrogen receptors; LUTS, lower urinary tract symptoms; PSA, prostate-specific antigen; E, serum estradiol; T, serum total testosterone; E/T, serum estradiol/total testosterone ratio; PVR, postvoid residual volume; PS, prostate size; Qmax, maximal flow rate; IPSS, International Prostate Symptom Score; SERα, stromal estrogen receptor alpha level; EERα, epithelial estrogen receptor alpha level; EERβ, epithelial estrogen beta level.
A p-value <0.05 was considered statistically significant.
*p<0.05. **p<0.01.
ACKNOWLEDGMENTS
We would like to thank all our patients for their cooperation and support in carrying out this study and we would also like to thank Mr. G.L Gupta for helping with the statistics.
References
1. Barry MJ. Epidemiology and natural history of benign prostatic hyperplasia. Urol Clin North Am. 1990; 17:495–507.
2. Castagnetta LA, Carruba G. Human prostate cancer: a direct role for oestrogens. In : Bock GR, Goode JA, editors. Non-reproductive actions of sex steroids. Chichester: John Wiley Sons;1995. p. 269–289.
3. Prins GS, Korach KS. The role of estrogens and estrogen receptors in normal prostate growth and disease. Steroids. 2008; 73:233–244.
4. Risbridger GP, Bianco JJ, Ellem SJ, McPherson SJ. Oestrogens and prostate cancer. Endocr Relat Cancer. 2003; 10:187–191.
5. Greene GL, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986; 231:1150–1154.
6. Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996; 392:49–53.
7. Tsurusaki T, Aoki D, Kanetake H, Inoue S, Muramatsu M, Hishikawa Y, et al. Zone-dependent expression of estrogen receptors alpha and beta in human benign prostatic hyperplasia. J Clin Endocrinol Metab. 2003; 88:1333–1340.
8. Schulze H, Claus S. Histological localization of estrogen receptors in normal and diseased human prostates by immunocytochemistry. Prostate. 1990; 16:331–343.
9. Brenner RM, West NB, McClellan MC. Estrogen and progestin receptors in the reproductive tract of male and female primates. Biol Reprod. 1990; 42:11–19.
10. Royuela M, de Miguel MP, Bethencourt FR, Sánchez-Chapado M, Fraile B, Arenas MI, et al. Estrogen receptors alpha and beta in the normal, hyperplastic and carcinomatous human prostate. J Endocrinol. 2001; 168:447–454.
11. Ehara H, Koji T, Deguchi T, Yoshii A, Nakano M, Nakane PK, et al. Expression of estrogen receptor in diseased human prostate assessed by non-radioactive in situ hybridization and immunohistochemistry. Prostate. 1995; 27:304–313.
12. Prins GS, Marmer M, Woodham C, Chang W, Kuiper G, Gustafsson JA, et al. Estrogen receptor-beta messenger ribonucleic acid ontogeny in the prostate of normal and neonatally estrogenized rats. Endocrinology. 1998; 139:874–883.
13. Mäkelä S, Strauss L, Kuiper G, Valve E, Salmi S, Santti R, et al. Differential expression of estrogen receptors alpha and beta in adult rat accessory sex glands and lower urinary tract. Mol Cell Endocrinol. 2000; 164:109–116.
14. Fixemer T, Remberger K, Bonkhoff H. Differential expression of the estrogen receptor beta (ERbeta) in human prostate tissue, premalignant changes, and in primary, metastatic, and recurrent prostatic adenocarcinoma. Prostate. 2003; 54:79–87.
15. Lau KM, LaSpina M, Long J, Ho SM. Expression of estrogen receptor (ER)-alpha and ER-beta in normal and malignant prostatic epithelial cells: regulation by methylation and involvement in growth regulation. Cancer Res. 2000; 60:3175–3182.
16. Fujimura T, Takahashi S, Urano T, Ogawa S, Ouchi Y, Kitamura T, et al. Differential expression of estrogen receptor beta (ERbeta) and its C-terminal truncated splice variant ERbetacx as prognostic predictors in human prostatic cancer. Biochem Biophys Res Commun. 2001; 289:692–699.
17. Nicholson TM, Sehgal PD, Drew SA, Huang W, Ricke WA. Sex steroid receptor expression and localization in benign prostatic hyperplasia varies with tissue compartment. Differentiation. 2013; 85:140–149.
18. Weihua Z, Warner M, Gustafsson JA. Estrogen receptor beta in the prostate. Mol Cell Endocrinol. 2002; 193:1–5.
19. Prins GS, Birch L, Couse JF, Choi I, Katzenellenbogen B, Korach KS. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor alpha: studies with alphaERKO and betaERKO mice. Cancer Res. 2001; 61:6089–6097.
20. Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000; 127:4277–4291.
21. Nicholson TM, Moses MA, Uchtmann KS, Keil KP, Bjorling DE, Vezina CM, et al. Estrogen receptor-α is a key mediator and therapeutic target for bladder complications of benign prostatic hyperplasia. J Urol. 2015; 193:722–729.
22. Allred DC, Bustamante MA, Daniel CO, Gaskill HV, Cruz AB Jr. Immunocytochemical analysis of estrogen receptors in human breast carcinomas. Evaluation of 130 cases and review of the literature regarding concordance with biochemical assay and clinical relevance. Arch Surg. 1990; 125:107–113.
23. Pasquali D, Staibano S, Prezioso D, Franco R, Esposito D, Notaro A, et al. Estrogen receptor beta expression in human prostate tissue. Mol Cell Endocrinol. 2001; 178:47–50.
24. Leav I, Lau KM, Adams JY, McNeal JE, Taplin ME, Wang J, et al. Comparative studies of the estrogen receptors beta and alpha and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am J Pathol. 2001; 159:79–92.
25. Shapiro E, Huang H, Masch RJ, McFadden DE, Wilson EL, Wu XR. Immunolocalization of estrogen receptor alpha and beta in human fetal prostate. J Urol. 2005; 174:2051–2053.
26. Buchanan DL, Kurita T, Taylor JA, Lubahn DB, Cunha GR, Cooke PS. Role of stromal and epithelial estrogen receptors in vaginal epithelial proliferation, stratification, and cornification. Endocrinology. 1998; 139:4345–4352.
27. Risbridger G, Wang H, Young P, Kurita T, Wang YZ, Lubahn D, et al. Evidence that epithelial and mesenchymal estrogen receptor-alpha mediates effects of estrogen on prostatic epithelium. Dev Biol. 2001; 229:432–442.
28. Kozák I, Bartsch W, Krieg M, Voigt KD. Nuclei of stroma: site of highest estrogen concentration in human benign prostatic hyperplasia. Prostate. 1982; 3:433–438.
29. Imamov O, Morani A, Shim GJ, Omoto Y, Thulin-Andersson C, Warner M, et al. Estrogen receptor beta regulates epithelial cellular differentiation in the mouse ventral prostate. Proc Natl Acad Sci U S A. 2004; 101:9375–9380.
30. Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998; 95:15677–15682.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download