Abstract
Bladder cancer remains the most immunogenic and expensive malignant tumor in the United States today. As the 4th leading cause of death from cancer in United States, Immunotherapy blocking immune checkpoints have been recently been applied to many aggressive cancers and changed interventions of urological cancers including advanced bladder cancer. The applied inhibition of PD-1–PD-L1 interactions can restore antitumor T-cell activity and enhance the cellular immune attack on antigens. The overall goals of this short review article are to introduce current cancer immunotherapy and immune checkpoint inhibitors, and to provide new insight into the underlying mechanisms that block immune checkpoints in tumor microenvironment. Furthermore, this review will address the preclinical and clinical trials to determine whether bladder cancer patients could benefit from this new cancer therapy in near future.
Cancer immuno-therapeutics or tumor immunotherapy in essence can be viewed as anticancer therapies to spark the body's immune system to help fight against cancer [12]. In the last several years, new insights into tumor immunology have lead to the development of a new class of drugs termed "immune checkpoint inhibitors"– several of which have demonstrated impressive antitumor responses in several malignancies, including melanoma, non-small cell lung cancer (NSCLC), and renal cell carcinoma (RCC). The personalized cancer immunotherapy (PCI) aims to provide each patient with a treatment tailored to harness his or her own immune system to fight cancer [34].
Currently, two representative tumor immunotherapies are in use in various cancer types–T-cell therapy and immune checkpoint blockade. The T-cell therapy is based on the specialized T cells produced by the immune system to target cancer cells, while immune checkpoint inhibition targets immune regulatory mechanisms and enhancing the immune system to attack cancer cells. These therapies are effective for some portion of patients with metastatic cancer. By blocking the PD-1–PD-L1 pathway, cancer cells become exposed and the immune system becomes triggered to send out the alerting messages and launch a system-wide attack on cancer cells [35].
Bladder cancer (BC) is the second most common urological malignancy in humans. There are an estimated 76,960 new cases of cancer in the urinary bladder every year in the United States (US). In 2016, there are expected to be 16,390 deaths resulting from BC, with the 5-year survival rate failing to improve significantly in the last 10 years. The clinico-pathologic feature classifies BC into 2 groups; nonmuscle invasive bladder cancer (NMIBC) and muscleinvasive bladder cancer (MIBC). MIBC is the main cause of cancer-specific deaths in BC patients [67]. NMIBC shows better survival than other malignancies, however, 30%–50% of patients with NMIBC will experience frequent recurrence after removing the primary tumor, and among them 10%–20% will progress to MIBC [89]. Therefore, frequent recurrence and eventual progression to MIBC have been challenges to patients and physicians. Unfortunately, there have been no new U.S. Food and Drug Administration (FDA)-approved therapies for those who cannot tolerate or fail to respond to cisplatin-based chemotherapy, a current gold standard treatment for BC [1011].
BC is highly immunogeneic cancer type with a higher rate of mutations, due to the fact that more mutations associate with a higher chance of tumor antigens triggering the correct immune response. The immune response of host to tumor cells is based on their interactions within the cancer microenvironment. There has been reported various types of tumor-infiltrating immune cells in BC, and how the signaling pathways between tumor and tumorinfiltrating immune cells. Immunotherapy has been used as a treatment for BC in the past. A portion of patients with moderate to high-grade BC—not those with muscle invasive BC—have been given intravesical immunotherapy with bacillus Calmette-Guérin (BCG) [121314]. BCG is the first U.S. FDA-approved immunotherapy in the US and reduces the risk of BC recurrence by stimulating an immune response. Resultantly, approximately 70% of BC patients go into remission after BCG therapy. Thus, the continued development of checkpoint inhibiting immunotherapies may provide a new treatment for advanced BC. Since the FDA granted atezolizumab (MPDL3280A, the anti-PD-L1 antibody) "breakthrough" status for the advanced and metastatic BC treatment in 2014, a couple of large immunotherapy clinical trials are on going for patients with BC. By blocking inhibitory molecules or, alternatively, activating stimulatory molecules, these treatments are designed to improve preexisting anticancer immune responses. Currently, there are a number of additional immune-based BC treatments using immune checkpoint inhibitors (such as pembrolizumab or atezolizumab) in development, which include nonmuscle invasive disease with BCG (clinical trial NCT02324582) as well as neo-adjuvant or adjuvant therapy after cystectomy (NCT02451423, NCT02450331). Numerous ongoing studies are expected to establish the worth of PD-1 pathway inhibitors in other tumor types as well as in combinations with approved agents.
In summary, this short review article will provide a general overview of the classical and current immune therapies for various cancer types. We will also discuss the clinical significance and impacts of immune checkpoint blockage for future BC management and treatment. Finally we will summarize the clinical trials currently on going for BC patients and potential side effects.
Cancer immunology is the study interaction between the immune system and cancer cells, contributing to the development of immuno-therapies such as vaccine or antibody therapies. The ultimate purpose of this study is the prevention of cancer initiation and disease progression [151617]. Immune therapy modulates and boosts the patient's immune response of the tumor leading to an anticancer response. Cancer immuno-surveillance and immuno-editing have been proposed as mechanisms by which tumors escape control through the development of tumor immunogenicity by the body's own immune system [1518].
BCG has been wildly used to treat BC, in particular to NMIBC, as a standard-of-care. The American Urological Association and European Association of Urology guidelines suggest that the first-line of treatment in the management of intermediate or high-risk NMIBC should complete transurethral resection of the tumor followed by BCG intravesical therapy [121314]. BCG therapy sparks the body's immune system and shows some positive effects, however, approximately 40% of patients show recurrence within 2 years. Population based studies also showed that BCG therapy remains suboptimal and unsuccessful [1920212223].
There are several inhibitory immune checkpoints between antigen presenting cells (APCs), T cells, cancer cells, and normal cells et al. T cells enter the active state when T-cell receptors bind to the major histocompatibility complex (MHC)-peptide complex on APCs or tumor cells. Several inhibitory checkpoints interact with their cognate ligands expressed on each respective tumor cell. When T cells encounter an antigen presented by MHC class I molecules on a cancer cell, the cancer cell expresses cognate ligands to interact with inhibitory checkpoints expressed on T cell. In general the immune checkpoint proteins are cell surface molecules on tumor-specific lymphocytes. There have been many advances in cancer immunology with immune checkpoints blockers against cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) and programmed death receptor ligand (PD-L1) et al. In next sections, we will further discuss immune therapies targeting these immune checkpoints.
To evade the host immune system, tumors use multiple strategies. In order to engage the immune system against tumors, the interactions between the checkpoint proteins and their ligands can be used to inhibit the proliferation and function of cancer cells. Antibodies targeting and blocking these immuno-inhibitory interactions have been suggested as a new immune therapy. Structurally homologous to the costimulatory molecule CD28, CTLA-4 exerts its inhibitory role by binding to the same ligands, B7.1 (CD80) and B7.2 (CD86) as CD28 does, but with a much higher affinity than CD28 [24-26]. This competitive binding inhibits CD28-induced T-cell activation and decreases cytokine production and cell cycle transition. CTLA-4 is expressed by Tregs, which plays an important role in peripheral tolerance via suppressive activity on cytotoxic T cells.
In 2011, the FDA approved ipilimumab (also called as Yervoy, an immune checkpoint blocking monoclonal antibody to target the CTLA-4) for metastatic melanoma patients [272829]. As one of the most well-known and well-studied members of the B7 super-family, ipilimumab is used as an adjuvant therapy in patients with melanoma in the skin and lymph nodes or patients whose diseases cannot be removed by surgery. Ipilimumab showed a prolonged survival in patients with advanced melanoma. Although ipilimumab can produce durable long-term responses in patients with advanced melanoma, it showed significant immune-related toxicities [18]. This therapeutic effect of CTLA-4 is being studied [30], not only in melanoma, but also in the treatment of other cancers, such as glioblastoma [313233]. Antitumor activity by enhancing naturally or vaccine induced T cells was also observed in animal studies, which provided the evidence that the transgenic adenocarcinoma of the mouse prostate-C1 is implantable in the tumor line. Furthermore, these findings led to testing whether CTLA-4 blockade effectively enhances immune response to reject tumors in men with prostate cancer in a clinical setting [343536].
Recent efforts demonstrated that antibodies blocking the PD-1 and PD-L1 pathway could have anti-tumor effects in the tumor microenvironment [3738]. PD-1 is a surface protein in the activated T cells, and is integral in basic protein function throughout the body. When PD-L1 or PD-L2 (ligands) binds to PD-1, the T cells become inactivated (Fig. 1). PD-L1 and the PD-1 pathway is involved in the T-cell immune evasion through the induction of T-cell apoptosis, anergy, functional exhaustion, or interleukin-10 (IL-10) production.
PD-1 is a type I transmembrane receptor member of the immunoglobulin superfamily, expressed by activated T cells, and binds to two ligands, PD-L1 (B7-H1, CD274) and PD-L2 (B7- DC, CD273)—both of which are part of the B7 immunoglobulin superfamily [394041]. PD-L1 is highly expressed in tumor cells, APCs, T lymphocytes, epithelial cells, or fibroblasts. PD-L1 secreted from PD-L1-overexpressing tumor cells protects them from CD8+ T-cell-mediated tumor cell lysis. Antibodies targeting either PD-1 or PD-L1 pathways reinvigorating the immune system showed clinically meaningful antitumor activity in patients with melanoma and NSCLC, RCC, BC and head and neck cancers. These antibodies showed the less immune-related toxicities, compared to ipilimumab. PD-1 targeting drugs include nivolumab (MDX-1106, BMS-936558, ONO-4538), pembrolizumab (MK-3475), and pidilizumab (CT-011) et al— all of which block PD-L1 from binding to PD-1, resulting in the T cell to continue active.
Nivolumab, which was approved by the FDA in December 2014, is a representative human IgG4 subtype anti-PD-1 monoclonal antibody that blocks ligand activation of the PD-1 receptor on activated T cells specific for tumor antigens. In the phase I studies, nivolumab was used with a single dose (0.3–10 mg/kg) for patients who no longer responded to other drugs. These treatment-refractory solid tumors include melanoma, colorectal cancer, castration-resistant prostate cancer, NSCLC, and RCC. The serum half-life of nivolumab ranged from 12 to 20 days. Nivolumab can be used alone or with other drugs such as ipilumumab and/or BRAF inhibitors for the patients with resectable or metastatic melanoma if they have no BRAF mutation. Advanced LSCLC or RCC patients who failed from cisplatin chemotherapy or angiogenesis inhibitor therapies along with patients suffering from Hodgkin's lymphoma, can be treated by nivolumab. Common treatment-related side effects such as pneumonitis, mild fatigue, diarrhea, pruritus, anorexia, rash, nausea, and decreased appetite were reported. Single-agent trials of nivolumab are ongoing or planned across a spectrum of tumor types including lymphomas, NSCLC, melanoma after progression on anti-CTLA-4 antibody, and hepatocellular carcinoma in multiple clinical trials including NCT02038946, NCT02038933, NCT01721759, NCT02066636, NCT02156804, and NCT01658878.
Pembrolizumab is a humanized monoclonal IgG4 antibody targeting PD-1 receptor. In the preclinical setting, antitumor activity of permbrolizumab was demonstrated in animal models of multiple tumor types. Being initially used to treat melanoma patients, pembrolizumab was approved in September 2014 by the FDA. Pembrolizumab was tested for treatment of advanced melanoma patients containing a BRAF mutation with ipilimumab and a BRAF inhibitor. The phase I study showed that the half-life of pembrolizumab is 13.6–21.7 days. This trial also showed the 37%–38% response rate in patients with advanced melanoma and an overall response rate of 26% in patients who had progressive disease after treatment with ipilimumab [42].
Phase II clinical trials of pembrolizumab were for NSCLC in patients with oligometastatic disease. Ongoing trials of pembrolizumab monotherapy are being conducted in patients with advanced solid tumors (NCT01295827), NSCLC (NCT01840579) and hematologic malignancies (NCT01953692). Randomized trials comparing pembrolizumab to standards of care are ongoing in PD-L1-positive NSCLC patients in comparison to combination chemotherapy (NCT02142738). Single-agent docetaxel in ipilimumab-treatment-naive patients with melanoma are also being tested in comparison to ipilimumab and ipilimumab-refractory patients with melanoma.
Pidilizumab is a humanized IgG1κ recombinant anti-PD-1 monoclonal antibody usable for the treatment of cancer and infectious diseases. In preclinical mouse cancer models as well as phase I study in patients with advanced hematologic cancers, pidilizumab has showed antitumor activity. The half-life of pidilizumab was observed very short with range of 217–410 hours. Phase II studies for diffuse large B-cell lymphoma, relapsed follicular lymphoma, or advanced melanoma, showed good results. However, the response rate of the solid tumor appeared to be less than those reported with the other anti-PD-1 inhibitors. Currently, the action mechanism of pidilizumab remains elusive.
Another approach to targeting the PD-1 pathway is through antibodies that bind to and prevent the activity of PD-L1—which is a 40 kDa-transmembrane protein expressed on activated T cells, B cells, and myeloid cells. PD-L1 binding to PD-1 contributes to T-cell inactivation through regulation of signaling pathways (e.g., NF-KB signaling). In animal models, a blockade of PD-1 has been tested for urine pancreatic carcinoma, B16 melanoma, squamous cell carcinoma, and CT26 colon carcinoma. PD-L1 targeting drugs such as BMS-936559, MPDL3280A, and MEDI-4736 et al. have been developed and applied to patients.
BMS-936559 is a fully humananized IgG4 antibody that inhibits binding of PD-L1 to PD-1 and CD80 with high affinity. MPDL3280A is also a human IgG1 antibody that targets PD-L1. A significant response rate was noted in patients with metastatic melanoma, RCC, NSCLC, or advanced BC in recent phase I studies using MPDL3280A. In particular, clinical trials for BC patients suggested that the PD-L1 expression in tumor-infiltrating immune cells was correlated with a response rate. As biomarkers were identified with treatment response, circulating interferon-γ, IL-18 and activated CD8+ T cells were suggested. Large scale phase II trial in patients with advanced BC is ongoing (NCT02108652) and supported by the FDA. We will address these efforts in the next session in great detail.
In addition to the targeting on PD-1/PD-L1 pathway, recent efforts blocking some of negative immune regulators have been accumulated to pursue the clinical application. These immune regulators include LAG-3 (lymphocyte-activation gene 3) [4344], TIM-3 (T-cell immunoglobulin and mucin containing protein-3) [4546], B7-H3 (B7 homolog 4, B7S1, B7x, VTCN1) [414748] and B7-H4/B7-Hx (V domain Ig suppressor of T-cell activation, B7-H5 or PD-1 Homolog) et al. [41495051]. Proteins such as CEACAM1 (carcinoembryonic antigen-related cell adhesion mole-cule 1 CD66a)—a transmembrane glycoprotein that negatively regulates cytotoxic T-cell proliferation—have been targeted. In melanoma, CEACAM1 monoclonal antibody blocks CEACAM1 homophilic interactions and inhibits cancer cells response to T cell mediated lysis [525354].
Examples of PD-1–PD-L1 pathway and other immune checkpoint inhibitors in clinical development were summarized in Fig. 2.
Advanced BC after recurrence is considered as one of the most difficult cancer types, with very low survival rates. No new treatment options for them have been suggested in the last 30 years (since 1998). The standard of care remains cisplatin-based chemotherapy, however, not all patients are eligible for this treatment. Cisplatin-based systemic chemotherapy administered in the neoadjuvant or adjuvant setting, often combined with radiotherapy, has decreased the morbidity and mortality from recurrent BC. Although these approaches are considered as a current gold standard for metastatic BC, treatment failure frequently occurs due to acquired chemoresistance. The ACS estimates that only 5%–15% 5 years survival rate was found after recurrence in people with advanced BC (stage IV), while approximately 88% of 5-year survival was shown when they were diagnosed in early phase (stage I).
In particular to BC, there are ongoing and planned trials of single-agent or combined inhibitors targeting multiple checkpoints as briefly described in the previous section. In an early-stage phase I trial, atezolizumab—which is designed to target PD-L1 expressing BC and tumor-infiltrating immune cells and inhibit the T-cell activation [5556]—achieved impressive results. There was an approximately 25% overall response rate in patients with PD-L1-positive metabstatic BC (as confirmed to have high levels of PD-L1 expression [e.g., score 2 or 3] by immunohistochemistry [IHC] analysis). Results in this study include that PD-L1 expressions were positively associated with better responses to atezolizumab. In order to evaluate PD-L1 expression on tumor cells and tumor-infiltrating immune cells, the investigational IHC test was developed by Roche Diagnostics. More than half of the patients with high levels of PD-L1 expression experienced tumor shrinkage at 12 weeks and survived at least one year after their treatment. Two of these patients (20%) had a complete response, with no signs of cancer after therapy. Notably, atezolizumab showed the favourable toxicity profile with no renal toxicity in BC patients who are generally old and have a higher incidence of renal impairment [5758].
Following the success of atezolizumab early trial, a randomized phase III study is ongoing to compare atezolizumab with standard-of-care chemotherapy in BC patients with relapse. Another study is used to compare atezolizumab effects in early-stage MIBC patients with high PD-L1 expression and BC patients are at risk for recurrence. All studies include the evaluation of an IHC test to determine PD-L1 expression status.
A recent Lancet paper reported results performed a by a single-arm, multicentre, phase 2 trial [59], which suggested that atezolizumab is effective in heavily pretreated patients with locally advanced or metastatic BC, and that response rates were significantly higher in patients with a greater expression of PD-L1 in tumor–infiltrating immune cells. This was the first report suggesting that the The Cancer Genome Atlas molecular subtypes are associated with atezolizumab response, and that mutation load is important to predict response to atezolizumab in advanced BC. These findings imply that genomic, molecular, and immunological factors are involved in the response rate, and support the idea that PD-L1 can be applicable as a biomarker to subclassify BC patients who are most likely to benefit when treated with atezolizumab or a combination of atezolizumab and another medicine. Table 1 summarizes the clinical trials.
More recent efforts have accumulated on the activation of antitumor immunity of tumor microenvironment using antibodies blocking the PD-1–PD-L1 pathway. In this short review article, we tried to address and summarize early stage clinical studies and clinical trials in various cancer types including advanced BC. There have been much efforts to develop combination regimens using PD-1 blockade as a backbone in unison with other chemotherapeutic drugs. Combination therapies to treat BC involving cytotoxic chemotherapy, antiangiogenic agents, alternative immune-checkpoint inhibitors, immuno-stimulatory cytokines and cancer vaccines are currently under clinical investigation. The combination treatment with checkpoint blockade and small molecule inhibitors is an attractive strategy since it would increases tumor antigen presentation.
For the PCI, another important research topic would be how to monitor the responses to immune therapy in patients. Possibly noninvasive (or minimally invasive) and accurate biomarkers should be urgently needed. However, we currently do not have gold standard biomarkers to predict the likelihood of response for each patient for immune checkpoint inhibitors. Efforts should be focused on identification of predictive biomarkers of responses, which may lead to advances in BC treatment and control.
Figures and Tables
Fig. 1
Tumor cells or tumor-infiltrating immune cells overexpress PD-L1 on their plasma membrane surface. PD-L1 binds to T-cell receptors (B7.1 or PD-1) in active T cells, deactivates cytotoxic T cells. Preventing PD-L1 from binding to its receptors on T cells makes T cells to remain active in tumor microenvironment.
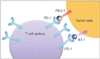
Table 1
Immune checkpoint inhibitor-based immunotherapies for bladder cancer

ACKNOWLEDGMENTS
The authors acknowledge support from National Institutes of Health grants (1U01DK103260, 1R01DK100974, U24 DK097154, NIH NCATS UCLA CTSI UL1TR000124 [to J.K.]), Department of Defense grants (PR140285 [to J.K.]), Centers for Disease Control and Prevention (1U01DP006079 [to J.K.]), and US-Egypt Science and Technology Joint Fund. IMAGINE NO IC Research Grant, the Steven Spielberg Discovery Fund in Prostate Cancer Research Career Development Award (to J.K.). J.K. is former recipient of Interstitial Cystitis Association Pilot Grant, a Fishbein Family IC Research Grant, New York Academy of Medicine, and Boston Children's Hospital Faculty Development.
References
1. Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004; 22:329–360.
2. Finn OJ. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol. 2012; 23:Suppl 8. viii6–viii9.
3. Yuan J, Hegde PS, Clynes R, Foukas PG, Harari A, Kleen TO, et al. Novel technologies and emerging biomarkers for personalized cancer immunotherapy. J Immunother Cancer. 2016; 4:3.
4. Hammerich L, Binder A, Brody JD. In situ vaccination: cancer immunotherapy both personalized and off-the-shelf. Mol Oncol. 2015; 9:1966–1981.
5. Nirschl CJ, Drake CG. Molecular pathways: coexpression of immune checkpoint molecules. Signaling pathways and implications for cancer immunotherapy. Clin Cancer Res. 2013; 19:4917–4924.
6. Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009; 374:239–249.
7. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108.
8. Messing E. Markers of detection. Urol Oncol. 2007; 25:344–347.
9. Quan C, Cha EJ, Lee HL, Han KH, Lee KM, Kim WJ. Enhanced expression of peroxiredoxin I and VI correlates with development, recurrence and progression of human bladder cancer. J Urol. 2006; 175:1512–1516.
10. Bellmunt J, Pons F, Orsola A. Molecular determinants of response to cisplatin-based neoadjuvant chemotherapy. Curr Opin Urol. 2013; 23:466–471.
11. Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012; 31:1869–1883.
12. Lenis AT, Chamie K. Bladder cancer in 2014: from the genomic frontier to immunotherapeutics. Nat Rev Urol. 2015; 12:74–76.
13. Macleod LC, Ngo TC, Gonzalgo ML. Complications of intravesical bacillus calmette-guerin. Can Urol Assoc J. 2014; 8(7-8):E540–E544.
14. Steinberg RL, Thomas LJ, Nepple KG. Intravesical and alternative bladder-preservation therapies in the management of non-muscle-invasive bladder cancer unresponsive to bacillus Calmette-Guerin. Urol Oncol. 2016; Jan. 14. Epub. DOI: 10.1016/j.urolonc.2015.12.004.
15. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012; 12:252–264.
16. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013; 39:1–10.
17. Lesterhuis WJ, Haanen JB, Punt CJ. Cancer immunotherapy: revisited. Nat Rev Drug Discov. 2011; 10:591–600.
18. Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006; 6:715–727.
19. Kamat AM, Sylvester RJ, Bohle A, Palou J, Lamm DL, Brausi M, et al. Definitions, end points, and clinical trial designs for non-muscle-invasive bladder cancer: recommendations from the international bladder cancer group. J Clin Oncol. 2016; Epub. DOI: 10.1200/JCO.2015.64.4070.
20. Liu S, Hou J, Zhang H, Wu Y, Hu M, Zhang L, et al. The evaluation of the risk factors for non-muscle invasive bladder cancer (NMIBC) recurrence after transurethral resection (TURBt) in Chinese population. PLoS One. 2015; 10:e0123617.
21. Matsumoto K, Gondo T, Hayakawa N, Maeda T, Ninomiya A, Nakamura S. The role of single instillation chemotherapy in patients who receive subsequent bacillus Calmette-Guerin: a retrospective single centre study, and systematic review of the literature. Can Urol Assoc J. 2015; 9(7-8):E411–E416.
22. O'Regan T, Tatton M, Lyon M, Masters J. The effectiveness of BCG and interferon against non-muscle invasive bladder cancer: a New Zealand perspective. BJU Int. 2015; 116:Suppl 3. 54–60.
23. Ahn JJ, Ghandour RA, McKiernan JM. New agents for bacillus Calmette-Guerin-refractory nonmuscle invasive bladder cancer. Curr Opin Urol. 2014; 24:540–545.
24. Hodi FS. Cytotoxic T-lymphocyte-associated antigen-4. Clin Cancer Res. 2007; 13(18 Pt 1):5238–5242.
25. Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996; 271:1734–1736.
26. Mokyr MB, Kalinichenko T, Gorelik L, Bluestone JA. Realization of the therapeutic potential of CTLA-4 blockade in lowdose chemotherapy-treated tumor-bearing mice. Cancer Res. 1998; 58:5301–5304.
27. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patientswith metastatic melanoma. N Engl J Med. 2010; 363:711–723.
28. Li Z, Chen L, Rubinstein MP. Cancer immunotherapy: are we there yet? Exp Hematol Oncol. 2013; 2:33.
29. Peggs KS, Quezada SA. Ipilimumab: attenuation of an inhibitory immune checkpoint improves survival in metastatic melanoma. Expert Rev Anticancer Ther. 2010; 10:1697–1701.
30. Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomized dose-comparison cohort of a phase 1 trial. Lancet. 2014; 384:1109–1117.
31. Brower V. Early-stage progress on glioma vaccines. J Natl Cancer Inst. 2011; 103:1361–1362.
32. Preusser M, Lim M, Hafler DA, Reardon DA, Sampson JH. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat Rev Neurol. 2015; 11:504–514.
33. Ribas A. Clinical development of the anti-CTLA-4 antibody tremelimumab. Semin Oncol. 2010; 37:450–454.
34. Kwek SS, Lewis J, Zhang L, Weinberg V, Greaney SK, Harzstark AL, et al. Preexisting levels of CD4 T cells expressing PD-1 are related to overall survival in prostate cancer patients treated with ipilimumab. Cancer Immunol Res. 2015; 3:1008–1016.
35. Reese Z, Straubhar A, Pal SK, Agarwal N. Ipilimumab in the treatment of prostate cancer. Future Oncol. 2015; 11:27–37.
36. Sobol I, Thompson RH, Dong H, Krco C, Kwon ED. Immunotherapy in prostate cancer. Curr Urol Rep. 2015; 16:34.
37. Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007; 19:813–824.
38. Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy. Inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012; 18:6580–6587.
39. Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002; 8:793–800.
40. Seliger B, Marincola FM, Ferrone S, Abken H. The complex role of B7 molecules in tumor immunology. Trends Mol Med. 2008; 14:550–559.
41. Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008; 8:467–477.
42. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014; 515:568–571.
43. Goldberg MV, Drake CG. LAG-3 in cancer immunotherapy. Curr Top Microbiol Immunol. 2011; 344:269–278.
44. Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, et al. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 1990; 171:1393–1405.
45. Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-γ-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011; 71:3540–3551.
46. Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005; 6:1245–1252.
47. Roth TJ, Sheinin Y, Lohse CM, Kuntz SM, Frigola X, Inman BA, et al. B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 2007; 67:7893–7900.
48. Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003; 4:899–906.
49. Seliger B, Quandt D. The expression, function, and clinical relevance of B7 family members in cancer. Cancer Immunol Immunother. 2012; 61:1327–1341.
50. Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci U S A. 2003; 100:10388–10392.
51. Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, et al. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci U S A. 2007; 104:19458–19463.
52. Gray-Owen SD, Blumberg RS. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol. 2006; 6:433–446.
53. Kuespert K, Pils S, Hauck CR. CEACAMs: their role in physiology and pathophysiology. Curr Opin Cell Biol. 2006; 18:565–571.
54. Thies A, Moll I, Berger J, Wagener C, Brummer J, Schulze HJ, et al. CEACAM1 expression in cutaneous malignant melanoma predicts the development of metastatic disease. J Clin Oncol. 2002; 20:2530–2536.
55. Taylor RC, Patel A, Panageas KS, Busam KJ, Brady MS. Tumor-infiltrating lymphocytes predict sentinel lymph node positivity in patients with cutaneous melanoma. J Clin Oncol. 2007; 25:869–875.
56. Sharma P, Shen Y, Wen S, Yamada S, Jungbluth AA, Gnjatic S, et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci U S A. 2007; 104:3967–3972.
57. Fenner A. Bladder cancer: could MPDL3280A offer a therapeutic breakthrough in metastatic bladder cancer? Nat Rev Urol. 2015; 12:61.
58. Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014; 515:558–562.
59. Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinumbased chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016; Mar. 4. Epub. DOI: 10.1016/S0140-6736(16)00561-4.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download