Abstract
Urothelial carcinomas of the urinary bladder have diverse biological and functional characteristics, and numerous factors are likely to be involved in recurrence, progression, and patient survival. While several molecular markers used to evaluate the development and prognosis of bladder cancer have been studied, they are of limited value; therefore, new molecular parameters useful for predicting the prognosis of bladder cancer patients (particularly patients at high risk of progression and recurrence) are required. Recent progress in the understanding of epigenetic modification and gene silencing has provided new opportunities for the detection, treatment, and prevention of cancer. Methylation is an important molecular mechanism in bladder cancer and may have utility as a prognostic and/or diagnostic marker. This review discusses the epigenetic issues involved in the detection and prediction of bladder cancer.
Bladder cancer (BC) is a heterogeneous disease; therefore, pathologically similar tumors may behave differently. In approximately 70% of all cases, patients present with nonmuscle invasive bladder cancer (NMIBC), whereas the remaining 30% present with muscle invasive bladder cancer (MIBC). The standard treatment for NMIBC is transurethral resection (TUR) complemented by intravesical immunotherapy or chemotherapy to prevent recurrence and progression [12]. Numerous factors are likely involved in disease outcome, and many patients with NMIBC experience disease recurrence and progression after primary treatment [12]. Furthermore, the potential of tumors to recur and progress to MIBC is highly unpredictable. Thus, more sensitive and noninvasive tumor markers that can detect and predict tumor recurrence, progression, and metastasis are required. Research efforts worldwide have focused on identifying clinically useful tumor markers or potentially valuable therapeutic targets to improve current diagnostic and management strategies for patients with BC [3]. Recent advances in our understanding of epigenetic modifications, including DNA methylation, histone modifications, and microRNAs, have provided new opportunities for detecting, treating, and preventing cancer. The utility of DNA methylation as a biomarker has attracted increasing attention in recent years because aberrant DNA methylation is a major characteristic of BC and plays a crucial role in tumor initiation and progression [456]. Here, we review the current knowledge base and epigenetic issues involved in the detection and prediction of BC.
While genetics refers to the study of information inherited on the basis of gene sequences, epigenetics is the study of reversible changes in gene function that can be inherited, or of other cell phenotypes that occur without any change in DNA sequence. DNA methylation occurs throughout the genome and involves the addition of a methyl group to the cytosine ring of the CpG dinucleotide [7]. The methylation pattern is established during development and is normally maintained throughout the life of an individual. Thus, DNA methylation is a key regulator of gene transcription and genomic stability, and inappropriately altered DNA methylation patterns are frequently detected as epigenetic changes in human cancers. Mechanisms that generally regulate normal DNA methylation patterns are impaired during tumorigenesis; therefore, many cancers show global hypomethylation, which is accompanied by regional hypermethylation in some promoter sequences. Aberrant methylation of tumor suppressor genes is the most well-categorized epigenetic change in human neoplasias [8]. Aberrant promoter methylation has been described for several genes in various malignant diseases, and each tumor type may have its own distinct pattern of methylation.
Because some epigenetic events occur early in the disease process, molecular diagnosis may facilitate detection before symptomatic or overt radiographic manifestations appear. Much progress has been made in the field of BC epigenetics research; examples are the biological characterization of methylation alterations and a move towards translational applications, including the development of potential new biomarkers for BC [456]. Because promoter hypermethylation is common in BC, potential DNA methylation markers for BC have been identified in serum, bladder washes, urine samples, and cancer tissues. Furthermore, methylation of these genes may facilitate cancer detection and/or correlate with a poor prognosis [456]. Thus, aberrant DNA methylation events may serve as biological markers for early detection, effective treatment, and accurate prognosis of BC.
Previous studies of DNA methylation-based biomarkers in BC focused on genes that are often methylated in other cancers. In 2001, the promoter methylation profiles of ten different cancer-related genes from 98 bladder tumors were examined to evaluate their relationship with clinicopathological features and the aggressiveness of the disease. Among these genes, four (RASSF1A, APC, CDH1, and CDH13) showed high rates of methylation (35%, 35%, 36%, and 29%, respectively) and these showed a significant correlation with various parameters associated with poor prognosis, such as tumor grade, growth pattern, muscle invasion, tumor stage, and ploidy status. A technique called methylation score (M score) analysis, which is based on a combination of methylation markers, was developed to increase the sensitivity/specificity of BC detection [9]. The methylation status of six Wnt-antagonist genes (sFRP-1, sFRP-2, sFRP-4, sFRP-5, Wif-1, and Dkk-3) was examined in BC tissues and corresponding normal bladder mucosa. The M score had a sensitivity of 77.2% and a specificity of 66.7% for BC detection, and yielded better results than analyses based on single genes. In addition, the M score was able to distinguish between superficial and invasive bladder tumors, with a sensitivity of 72.2% and a specificity of 61.1%, respectively, making it a useful staging biomarker.
The evolution of classic single-gene DNA methylation detection assays into genome-wide microarray based technologies, coupled with the development of cutting-edge bioinformatics approaches, has provided an unprecedented opportunity to investigate the role of aberrant DNA methylation in the genesis and progression of BC. Several high-throughput screening methods have been developed to simultaneously analyze the methylation status of hundreds of preselected genes using universal bead arrays. These methods have led to the discovery of methylation signatures that distinguish normal tissue from cancer tissue. Wolff et al. [10] used the GoldenGate methylation assay (Illumina, San Diego, CA, USA), which comprises 1,370 CpG sites, to study methylation patterns in 49 samples from patients with NMIBC, 38 from those with MIBC (with matched normal-appearing urothelium), and 12 samples of urothelia from age-matched cancer-free controls with no history of urothelial cancer. They found distinct patterns of hypomethylation in NMIBC and widespread hypermethylation in MIBC, confirming that the two pathways differ epigenetically as well as genetically. Relative to control samples from urothelial cancer-free patients, invasive tumors had 526 hypermethylated loci (38%) and noninvasive tumors had 132 hypermethylated loci (10%), of which 117 (89%) overlapped with those found in the invasive tumors. Normal-appearing urothelia samples taken from sites located at least 5 cm away from the invasive tumor had 169 hypermethylated loci (12%), of which 142 (89%) were the same as those found in the invasive tumor. The authors concluded that these patterns were indicative of an epigenetic 'field defect', i.e., methylation was already present in normal-looking cells before the onset of tumorigenesis. This finding suggests that methylation precedes tumorigenesis, which may have implications for the surveillance of patients by urine testing because methylation will presumably persist in the normal urothelium after tumor resection.
Currently, cancer recurrence or progression in BC patients is monitored by periodic cystoscopy and urine cytology, the frequency of which varies according to the risk factors associated with the disease. Although cystoscopic examination is the gold standard for BC diagnosis, it is costly, involves substantial patient discomfort, and has variable sensitivity. Moreover, the sensitivity of cytological analysis is low, particularly for low-grade transitional cell carcinomas, and its accuracy depends on the pathologist's experience. Frequent recurrence of BC after TUR and its subsequent progression are problems for both patients and urologists. The challenge for the clinician is to develop reasonable surveillance protocols that facilitate cost-effective and noninvasive monitoring. To date, molecular biology and genetic studies have identified several potential markers in serum, bladder washes, urinary specimens, and cancer tissues. However, the limitations of currently available markers have increased interest in identifying other molecular parameters that provide a more accurate prognosis for BC patients. Of particular interest is the epigenetic silencing of tumor suppressor genes.
Cancer detection via abnormal DNA methylation is quite powerful due to the inherent stability of DNA compared with that of RNA or proteins. Because promoter hypermethylation is a frequent occurrence in BC, detecting cancer-specific hypermethylation events in various biological fluids (urine/blood) and tissues would be feasible as these biological materials are easily accessible. Chan et al. [11] were the first to demonstrate the feasibility of diagnosing BC by detecting methylated DNA in voided urine. They examined the methylation status of 7 genes (RARβ, DAPK, E-cadherin, p16, p15, GSTP1, and MGMT) in 22 voided urine samples from BC patients and 17 from age- and sex-matched controls. A panel comprising some of these markers (DAPK, RARβ, E-cadherin, and p16) achieved a sensitivity of 91% and a specificity of 76% for detecting BC; by comparison, cytology achieved a sensitivity and specificity of 46% and 100%, respectively. Examination of matched tumor and urine samples identified no false positive urine samples when the tumor was negative for methylation, indicating that these markers were specific. The feasibility of detecting DNA methylation or hypermethylation in voided urine, and its potential role as a tumor marker for BC, have since been examined in several studies (Table 1) [9111213141516171819202122232425262728293031323334353637383940414243].
Friedrich et al. [14] examined DNA methylation of apoptosis-associated genes in urine sediments. DAPK methylation was detected in 22% of samples (8 of 37), TERT methylation in 51% (18 of 37), and BCL2 methylation in 65% (24 of 37). Combined methylation analyses (i.e., DAPK, BCL2, and TERT) yielded both high sensitivity (78%) and specificity (100%) for detecting BC. Similarly, Hoque et al. [13] examined the potential of detecting DNA hypermethylation in voided urine and its promising role as a tumor marker for BC. They used a quantitative real-time PCR assay to examine promoter hypermethylation in DNA present in urine sediments obtained from 175 BC patients and 94 age-matched control subjects. Nine genes were examined: APC, p14ARF, CDH1, GSTP1, MGMT, p14ARF, RARb2, RASSF1A, and TIMP3. Combined methylation analysis based on four genes (p14ARF, p14ARF, MGMT, and GSTP1) yielded 69% sensitivity and 100% specificity. More recently, Renard et al. [23] reported that methylated TWIST1 and NID2 genes were promising urine markers for BC based on a well-designed study approach to selecting and validating candidate genes. BC cell lines and BC-related patient samples were used to select the best candidate markers, which were then validated in methylation-specific polymerase chain reaction assays using 496 urine samples collected from three urology clinical sites. They identified two genes, TWIST1 and NID2, that were frequently methylated in urine samples collected from BC patients, including those with early-stage and low-grade disease. The sensitivity of this 2-gene panel (90%) was significantly better than that of cytology (48%), with comparable specificity (93% and 96%, respectively). The positive predictive value (PPV) and negative predictive value of the 2-gene panel was 86% and 95%, respectively. The clinical feasibility of TWIST1 and NID2 as urinary biomarkers for detecting BC were recently evaluated; unfortunately, the different studies yielded different values for the sensitivity and specificity of TWIST1 and NID2 for detecting BC [394042]. Yegin et al. [39] examined the methylation patterns of TWIST1 and NID2 genes in urine samples from 24 BC patients and 15 controls. Methylation of TWIST1 and NID2 was detected in 87.5% and 95.8%, respectively, of samples. The sensitivity of TWIST1 and NID2 gene methylation (87.5% and 95.8%, respectively) for cancer detection was similar to that reported by Renard et al. [23], and higher than that of urine cytology (62.5%). Abern et al. [40] examined urine samples from 111 BC patients in an attempt to externally validate a urine-based methylation assay that combined TWIST1 and NID2. When samples were examined in accordance with the assay described by Renard et al. [23], the sensitivity and specificity were 79% and 63%, respectively; however, when optimized for the 111 samples examined, the sensitivity and specificity were 75% and 71%, respectively. Fantony et al. [42] re-examined the diagnostic utility of the TWIST1/NID2 gene methylation assay by using it to externally validate 209 urine samples obtained from BC patients. They found the results to be poor. Reinert et al. [29] evaluated the clinical utility of methylation markers (selected from genome-voided microarrays) in urine samples from 119 BCs and 59 controls. They found that a 4-marker panel (ZNF154, HOXA9, POU4F2, and EOMES) achieved a sensitivity of 84% and a specificity of 96% for detecting BC. A validation study based on DNA obtained from 184 BC patients and 35 controls showed that a panel of 6 methylation markers (EOMES, HOXA9, POU4F2, TWIST1, VIM, and ZNF154) had a sensitivity of 82%–89% and a specificity of 94%–100% for detecting BC [34]. In addition, these methylation markers predicted recurrence within a 12 month follow-up period with a sensitivity of 88–94% and a specificity of 43%–67%. Recently, Su et al. [41] reported changes in the levels of urinary methylation markers in 368 urine samples serially collected from 90 NMIBC patients. They showed that a panel of 3 markers (SOX1, IRAK3, and L1-MET) discriminated between patients with and without recurrence (with a sensitivity and specificity of 86%/89% and 80%/97% in the test and validation sets, respectively). This panel provided better resolution than either cytology or cystoscopy for the detection of early recurrence.
In summary, modern techniques for examining DNA methylation permit the sensitive and quantitative detection of methylated genes, with impressive results. However, methylation markers for BC diagnosis are still not as well-established as U.S. Food and Drug Administrationapproved markers. Most reported markers have been tested on cohorts that varied greatly between studies. In addition, many markers lack validation in independent cohorts with predetermined cutoff values. Independent validation experiments often achieve lower sensitivity and/or specificity values because the cutoff values are only fitted to data obtained in the initial experiment. Nevertheless, it is evident that methylation markers are more sensitive than cytology, and that some markers show specificity comparable with that of cytology. Only a highly selective panel of methylation markers will increase the sensitivity and specificity of urine analysis in the clinic. In addition, future studies should use standardized assays and cutoff values to compare DNA methylation markers with established markers in a large-scale well-designed prospective cohort.
Several studies show a positive association between the hypermethylation status of genes and a poor prognosis for BC patients; indeed, some of these genes were identified as independent predictive factors of BC prognosis [152544454647484950515253545556575859606162636465666768697071] (Table 2). Maruyama et al. [50] were the first to report methylation-based prognostic markers in BC. They determined the methylation status of ten genes in 98 BC specimens and calculated the methylation index (MI). Of these ten genes, the methylation status of two (CDH1 and FHIT), and the MI, were significantly correlated with poor survival. However, CDH1 methylation was only an independent risk factor for poor survival. Tada et al. [48] demonstrated that an increased rate of DAPK methylation in BC specimens was significantly associated with a reduced time to recurrence. After adjusting for stage and grade, DAPK methylation was the most important prognostic factor for recurrence. Likewise, a study examining the methylation status of laminin-5-encoding genes showed that LAMC2 methylation was strongly associated with poor survival [15]. Catto et al. [51] analyzed hypermethylation at 11 CpG islands in a large cohort of urothelial carcinomas. Compared with unmethylated tumors, methylation at these sites was significantly associated with advanced grade and stage. Hypermethylation of RASSF1A and DAPK were independent prognostic markers for progression of NMIBC. Friedrich et al. [46] examined the methylation status of 20 cancer-associated genes in 105 consecutive primary NMIBC patients. Among these genes, the methylation status of six (SOCS-1, STAT-1, BCL-2, DAPK, TIMP-3, and CDH1) was associated with tumor recurrence [46]. However, only TIMP-3 was significantly associated with prolonged recurrence-free survival. Christoph et al. [44] reported that APAF-1 and IGFBP-3 methylation was an independent prognostic marker for recurrence in NMIBC. A genome-wide study by Kandimalla et al. [60] revealed that the methylation status of TBX2, TBX3, GATA2, and ZIC4 was associated with cancer progression in both test and validation sets of pTa tumors. However, multivariate analysis identified only TBX3 and GATA2 methylation as an independent predictor of progression when compared with other clinicopathological variables. A study of 181 BC patients identified the methylation of HOXA9, ISL1, and ALDH1A3 as an independent predictor of disease recurrence and progression [63]. Sacristan et al. [68] classified paraffin-embedded samples from 251 primary NMIBC patients into subgroups (pTa low-grade [LG], n=79; pT1LG, n=81; and pT1 high-grade [HG], n=91) according to the methylation status of 25 tumor suppressor genes, and examined whether this could be used to predict the outcome. They found that methylation of RARB, CD44, PAX5A, GSTP1, IGSF4 (CADM1), PYCARD, CDH13, TP53, and GATA5 distinguished pTa from pT1 tumors, whereas RARB, CD44, GSTP1, IGSF4, CHFR, PYCARD, TP53, STK11, and GATA5 distinguished LG from HG tumors. Multivariate analyses indicated that methylation of PAX5A, WT1, and BRCA1 was an independent predictor of recurrence in pTaLG, that methylation of PAX6, ATM, CHFR, and RB1 was an independent predictor of recurrence in pT1LG disease, and that methylation of PYCARD was an independent predictor of recurrence in pT1HG disease. Methylation of PAX5A and RB1 was an independent predictor of recurrence overall.
A significant association between hypermethylation of genes and poor survival has been reported for BC [49545559616569]. Yates et al. [54] examined 17 gene promoters in 96 malignant urothelial samples. Multivariate analysis revealed that the overall degree of methylation was more significantly associated with subsequent progression and death than tumor stage. Furthermore, epigenetic predictive models developed using artificial intelligence techniques identified the presence and timing of tumor progression with 97% specificity and 75% sensitivity. A study of 101 BC samples (56 NMIBC and 45 MIBC) showed that methylation of SOX9 was significantly associated with poor overall survival [55]. Kim et al. [49] examined the association between RUNX3 inactivation and BC over a 50-month median follow-up period. Multivariate Cox regression analyses revealed that RUNX3 hypermethylation was the only strong predictor of BC progression, and that the methylation status of RUNX3 was significantly associated with cancer-specific survival. Cebrian et al. [59] examined the methylation status of KISS1 in 804 paraffin-embedded BC specimens. KISS1 methylation was associated with increasing stage, tumor grade, and poor disease-specific survival. A study of 133 BC patients found that methylation of CDH 13 was signif icantly associated with tumor recurrence and a poor prognosis. In addition, multivariate analysis indicated that CDH 13 was independently associated with poor outcome and the relative risk of death [61]. Garcia-Baquero et al. [65] examined the methylation status of 18 genes in paraffin-embedded primary bladder tumors (n=61) and identified prognostic indicators of recurrence (SFRP5 and H2AFX), progression (CACNA1G), and disease-specific survival (SFRP5). A recent study of PCDH17 promoter methylation in BC revealed an association between a significant reduction in survival and an independent predictor of overall survival [69].
Although data are sparse, several studies identified a significant association between methylation status and predicted responses to bacillus Calmette-Guérin (BCG) in high-risk BC patients [575864]. Alvarez-Mugica et al. [57] examined myopodin methylation in 170 T1G3 BC specimens, including a subset of 108 patients who underwent BCG treatment. Univariate and multivariate analyses revealed that myopodin methylation was associated with an increased rate of recurrence and progression, and with shorter disease-specific overall survival. In the subset of patients treated with BCG, myopodin methylation was also associated with increased recurrence and progression, and with shorter disease-specific survival, with PPVs of 38.3%, 25.9%, and 14.8%, respectively. Agundez et al. [58] examined the methylation status of 25 tumor suppressor genes and its utility for predicting BCG responses in 91 patients with T1G3 high-risk BCs. Multivariate analysis identified a combination of MSH6 and THBS1 as the best predictor of progression. Similarly, another study examined the utility of PMF-1 methylation for predicting the clinical outcome of 108 T1HG NMIBC patients receiving treatment with BCG [64]. Multivariate analysis identified PMF-1 methylation as being associated with recurrence and progression. The methylation status of these genes may serve to distinguish high-risk patients that respond to BCG from those who may require more aggressive therapeutic approaches.
In summary, DNA methylation is significantly associated with advanced stage, high rates of tumor progression, poor responses to BCG therapy, and increased mortality. Thus, methylation status may be useful as a prognostic marker for BC. However, numerous factors are involved in progression and survival, and future studies should perform multivariate analyses on large numbers of patients; also, long-term follow-up is needed to confirm that the methylation status is independent of other variables. Moreover, understanding the epigenetic changes that occur during the early steps of cancer progression may improve molecular strategies aimed at cancer prevention and/or early intervention.
Epigenetic alterations can also be identified in body fluids such as blood. Live, apoptotic, and necrotic tumor cells shed into circulation may be a source of measurable epigenetic biomarkers [72]. Relatively few studies have reported the identification of blood-based BC methylation markers [4556737475767778] (Table 3). Dominguez et al. [45] were the first to describe the presence of methylated DNA in plasma samples from 27 BC patients. Of these, p14ARF and p16INK4a promoter methylation was detected in 87% and 40%, respectively, of samples. Hypermethylation of p14ARF in plasma was significantly associated with multicentric foci, larger tumors, and relapse. Valenzuela et al. [73] examined the methylation status of p16INK4a in serum samples from 86 BC patients and 49 controls, and showed sensitivity, specificity, and PPV for the detection of BC of 0.226, 0.95, and 0.98, respectively. Another study showed that the frequency of p16INK4a and DAPK methylation in serum was 45% and 64.3%, respectively [74]. Ellinger et al. [56] studied the methylation status of APC, DAPK, GSTP1, PTGS2, TIG1, and Reprimo in the serum of 45 BC patients and 45 controls. Hypermethylation at APC, GSTP1, or TIG1 distinguished BC from controls with 80% sensitivity and 93% specificity. Hypermethylation correlated significantly with prognostically unfavorable clinicopathological parameters, and APC hypermethylation was significantly associated with cancer-specific mortality. Recently, Hauser et al. [75] analyzed the DNA hypermethylation patterns of APC, GSTP1, p14, p16, RARB, RASSF1A, and TIMP3 in a prospective, multicenter cohort (n=227). They found that both the methylation level at each gene site and the number of methylated genes was higher in BC patients than in healthy individuals; however, levels between BC patients and patients with nonmalignant disease were similar. The sensitivity and specificity of methylated genes for discriminating BC patients from healthy individuals were 62% and 89%, respectively. DNA hypermethylation did not correlate with advanced stage or grade in BC patients. The authors concluded that DNA methylation status has limited value as a biomarker in patients with noninvasive BC.
Several serum methylation markers (CDH13, PCDH10, and PCDH17) are prognostic indicators for BC [767778]. Although the frequency of CDH13 (30%, n=127 [76]), PCDH10 (50%, n=117 [77]) and PCDH17 (52%, n=151 [78]) is low, none have been detected in control samples. Moreover, the methylation pattern of CDH13, PCDH10, and PCDH17 is significantly associated with aggressive tumor characteristics (tumor size, stage, and grade), and PCDH10 and PCDH17 are independent predictors of cancer-specific survival [767778].
In summary, hypermethylated genes can be detected in the blood, but the rate of methylation is relatively low and widely variable. Few data are available regarding the prognostic value of blood-based hypermethylation markers in BC. Thus, the clinical relevance of blood-based hypermethylation markers may remain limited; however, future studies should shed light on its clinical value.
It is clear that much has been discovered about the molecular events that underlie promoter methylation and its role in BC detection, recurrence, progression, and survival. However, the majority of studies have simply identified potential markers; what is now needed is for these markers to be translated to the clinic. Rigorous multicenter prospective validation studies involving large cohorts in a large clinical setting should be performed along with robust statistical analyses. Cross talk between different molecular pathways and tumor heterogeneity mean that a single methylation marker would be of limited value for predicting disease status and outcome. These weaknesses might be overcome by genome-wide association studies. Nevertheless, our understanding of the epigenetic events that lead to urothelial tumorigenesis and prognosis is improving, and should allow clinicians to identify key epigenetic changes that can be targeted for detecting and predicting disease. Methylation markers in BC will be valuable tools for stratifying heterogeneous BC patient populations into risk groups, which can then be used to guide clinical decision-making (e.g., observation versus adjuvant therapy). Aberrant patterns of epigenetic modification could be crucial parameters for BC diagnosis, prognosis, and therapy.
ACKNOWLEDGMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2015R1D1A1A01057786) and Ministry of Science, ICT and Future Planning (NRF-2014R1A2A1 A09006983).
References
1. Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Comperat E, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013; 64:639–653. PMID: 23827737.

2. Kamat AM, Hegarty PK, Gee JR, Clark PE, Svatek RS, Hegarty N, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Screening, diagnosis, and molecular markers. Eur Urol. 2013; 63:4–15. PMID: 23083902.

3. Cheng L, Davison DD, Adams J, Lopez-Beltran A, Wang L, Montironi R, et al. Biomarkers in bladder cancer: translational and clinical implications. Crit Rev Oncol Hematol. 2014; 89:73–111. PMID: 24029603.

4. Kim WJ, Kim YJ. Epigenetic biomarkers in urothelial bladder cancer. Expert Rev Mol Diagn. 2009; 9:259–269. PMID: 19379084.

5. Besaratinia A, Cockburn M, Tommasi S. Alterations of DNA methylome in human bladder cancer. Epigenetics. 2013; 8:1013–1022. PMID: 23975266.

6. Kandimalla R, van Tilborg AA, Zwarthoff EC. DNA methylation-based biomarkers in bladder cancer. Nat Rev Urol. 2013; 10:327–335. PMID: 23628807.

8. Laird PW. The power and the promise of DNA methylation markers. Nat Rev Cancer. 2003; 3:253–266. PMID: 12671664.

9. Urakami S, Shiina H, Enokida H, Kawakami T, Kawamoto K, Hirata H, et al. Combination analysis of hypermethylated Wntantagonist family genes as a novel epigenetic biomarker panel for bladder cancer detection. Clin Cancer Res. 2006; 12(7 Pt 1):2109–2116. PMID: 16609023.

10. Wolff EM, Chihara Y, Pan F, Weisenberger DJ, Siegmund KD, Sugano K, et al. Unique DNA methylation patterns distinguish noninvasive and invasive urothelial cancers and establish an epigenetic field defect in premalignant tissue. Cancer Res. 2010; 70:8169–8178. PMID: 20841482.

11. Chan MW, Chan LW, Tang NL, Tong JH, Lo KW, Lee TL, et al. Hypermethylation of multiple genes in tumor tissues and voided urine in urinary bladder cancer patients. Clin Cancer Res. 2002; 8:464–470. PMID: 11839665.
12. Dulaimi E, Uzzo RG, Greenberg RE, Al-Saleem T, Cairns P. Detection of bladder cancer in urine by a tumor suppressor gene hypermethylation panel. Clin Cancer Res. 2004; 10:1887–1893. PMID: 15041703.

13. Hoque MO, Begum S, Topaloglu O, Chatterjee A, Rosenbaum E, Van Criekinge W, et al. Quantitation of promoter methylation of multiple genes in urine DNA and bladder cancer detection. J Natl Cancer Inst. 2006; 98:996–1004. PMID: 16849682.

14. Friedrich MG, Weisenberger DJ, Cheng JC, Chandrasoma S, Siegmund KD, Gonzalgo ML, et al. Detection of methylated apoptosis-associated genes in urine sediments of bladder cancer patients. Clin Cancer Res. 2004; 10:7457–7465. PMID: 15569975.

15. Sathyanarayana UG, Maruyama R, Padar A, Suzuki M, Bondaruk J, Sagalowsky A, et al. Molecular detection of noninvasive and invasive bladder tumor tissues and exfoliated cells by aberrant promoter methylation of laminin-5 encoding genes. Cancer Res. 2004; 64:1425–1430. PMID: 14973053.

16. Chan MW, Chan LW, Tang NL, Lo KW, Tong JH, Chan AW, et al. Frequent hypermethylation of promoter region of RASSF1A in tumor tissues and voided urine of urinary bladder cancer patients. Int J Cancer. 2003; 104:611–616. PMID: 12594816.
17. Yates DR, Rehman I, Meuth M, Cross SS, Hamdy FC, Catto JW. Methylational urinalysis: a prospective study of bladder cancer patients and age stratified benign controls. Oncogene. 2006; 25:1984–1988. PMID: 16288222.

18. Yu J, Zhu T, Wang Z, Zhang H, Qian Z, Xu H, et al. A novel set of DNA methylation markers in urine sediments for sensitive/specific detection of bladder cancer. Clin Cancer Res. 2007; 13:7296–7304. PMID: 18094410.

19. Aleman A, Cebrian V, Alvarez M, Lopez V, Orenes E, Lopez-Serra L, et al. Identification of PMF1 methylation in association with bladder cancer progression. Clin Cancer Res. 2008; 14:8236–8243. PMID: 19088041.

20. Cebrian V, Alvarez M, Aleman A, Palou J, Bellmunt J, Gonzalez-Peramato P, et al. Discovery of myopodin methylation in bladder cancer. J Pathol. 2008; 216:111–119. PMID: 18636402.

21. Costa VL, Henrique R, Danielsen SA, Duarte-Pereira S, Eknaes M, Skotheim RI, et al. Three epigenetic biomarkers, GDF15, TMEFF2, and VIM, accurately predict bladder cancer from DNA-based analyses of urine samples. Clin Cancer Res. 2010; 16:5842–5851. PMID: 20975101.

22. Lin HH, Ke HL, Huang SP, Wu WJ, Chen YK, Chang LL. Increase sensitivity in detecting superficial, low grade bladder cancer by combination analysis of hypermethylation of E-cadherin, p16, p14, RASSF1A genes in urine. Urol Oncol. 2010; 28:597–602. PMID: 19181545.

23. Renard I, Joniau S, van Cleynenbreugel B, Collette C, Naome C, Vlassenbroeck I, et al. Identification and validation of the methylated TWIST1 and NID2 genes through real-time methylation-specific polymerase chain reaction assays for the non-invasive detection of primary bladder cancer in urine samples. Eur Urol. 2010; 58:96–104. PMID: 19674832.

24. Cabello MJ, Grau L, Franco N, Orenes E, Alvarez M, Blanca A, et al. Multiplexed methylation profiles of tumor suppressor genes in bladder cancer. J Mol Diagn. 2011; 13:29–40. PMID: 21227392.

25. Chen PC, Tsai MH, Yip SK, Jou YC, Ng CF, Chen Y, et al. Distinct DNA methylation epigenotypes in bladder cancer from different Chinese sub-populations and its implication in cancer detection using voided urine. BMC Med Genomics. 2011; 4:45. PMID: 21599969.

26. Chung W, Bondaruk J, Jelinek J, Lotan Y, Liang S, Czerniak B, et al. Detection of bladder cancer using novel DNA methylation biomarkers in urine sediments. Cancer Epidemiol Biomarkers Prev. 2011; 20:1483–1491. PMID: 21586619.

27. Costa VL, Henrique R, Danielsen SA, Eknaes M, Patricio P, Morais A, et al. TCF21 and PCDH17 methylation: an innovative panel of biomarkers for a simultaneous detection of urological cancers. Epigenetics. 2011; 6:1120–1130. PMID: 21847011.
28. Dudziec E, Goepel JR, Catto JW. Global epigenetic profiling in bladder cancer. Epigenomics. 2011; 3:35–45. PMID: 22126151.

29. Reinert T, Modin C, Castano FM, Lamy P, Wojdacz TK, Hansen LL, et al. Comprehensive genome methylation analysis in bladder cancer: identification and validation of novel methylated genes and application of these as urinary tumor markers. Clin Cancer Res. 2011; 17:5582–5592. PMID: 21788354.

30. Serizawa RR, Ralfkiaer U, Steven K, Lam GW, Schmiedel S, Schuz J, et al. Integrated genetic and epigenetic analysis of bladder cancer reveals an additive diagnostic value of FGFR3 mutations and hypermethylation events. Int J Cancer. 2011; 129:78–87. PMID: 20824703.

31. Vinci S, Giannarini G, Selli C, Kuncova J, Villari D, Valent F, et al. Quantitative methylation analysis of BCL2, hTERT, and DAPK promoters in urine sediment for the detection of nonmuscle-invasive urothelial carcinoma of the bladder: a prospective, two-center validation study. Urol Oncol. 2011; 29:150–156. PMID: 19272801.

32. Berrada N, Amzazi S, Ameziane El, Benbacer L, El Mzibri M, Khyatti M, et al. Epigenetic alterations of adenomatous polyposis coli (APC), retinoic acid receptor beta (RARβ) and survivin genes in tumor tissues and voided urine of bladder cancer patients. Cell Mol Biol (Noisy-le-grand). 2012; (Suppl. 58):OL1744–OL1751. PMID: 22992440.
33. Eissa S, Zohny SF, Shehata HH, Hegazy MG, Salem AM, Esmat M. Urinary retinoic acid receptor-β2 gene promoter methylation and hyaluronidase activity as noninvasive tests for diagnosis of bladder cancer. Clin Biochem. 2012; 45:402–407. PMID: 22286019.

34. Reinert T, Borre M, Christiansen A, Hermann GG, Orntoft TF, Dyrskjot L. Diagnosis of bladder cancer recurrence based on urinary levels of EOMES, HOXA9, POU4F2, TWIST1, VIM, and ZNF154 hypermethylation. PLoS One. 2012; 7:e46297. PMID: 23056278.

35. Scher MB, Elbaum MB, Mogilevkin Y, Hilbert DW, Mydlo JH, Sidi AA, et al. Detecting DNA methylation of the BCL2, CDKN2A and NID2 genes in urine using a nested methylation specific polymerase chain reaction assay to predict bladder cancer. J Urol. 2012; 188:2101–2107. PMID: 23083854.
36. Zhao Y, Guo S, Sun J, Huang Z, Zhu T, Zhang H, et al. Methylcap-seq reveals novel DNA methylation markers for the diagnosis and recurrence prediction of bladder cancer in a Chinese population. PLoS One. 2012; 7:e35175. PMID: 22529986.

37. Zuiverloon TC, Beukers W, van der Keur KA, Munoz JR, Bangma CH, Lingsma HF, et al. A methylation assay for the detection of non-muscle-invasive bladder cancer (NMIBC) recurrences in voided urine. BJU Int. 2012; 109:941–948. PMID: 21756281.

38. Chihara Y, Kanai Y, Fujimoto H, Sugano K, Kawashima K, Liang G, et al. Diagnostic markers of urothelial cancer based on DNA methylation analysis. BMC Cancer. 2013; 13:275. PMID: 23735005.

39. Yegin Z, Gunes S, Buyukalpelli R. Hypermethylation of TWIST1 and NID2 in tumor tissues and voided urine in urinary bladder cancer patients. DNA Cell Biol. 2013; 32:386–392. PMID: 23682613.
40. Abern MR, Owusu R, Inman BA. Clinical performance and utility of a DNA methylation urine test for bladder cancer. Urol Oncol. 2014; 32:51.e21–51.e26. PMID: 24360662.

41. Su SF, de Castro Abreu AL, Chihara Y, Tsai Y, Andreu-Vieyra C, Daneshmand S, et al. A panel of three markers hyper- and hypomethylated in urine sediments accurately predicts bladder cancer recurrence. Clin Cancer Res. 2014; 20:1978–1989. PMID: 24691641.

42. Fantony JJ, Abern MR, Gopalakrishna A, Owusu R, Jack Tay K, Lance RS, et al. Multi-institutional external validation of urinary TWIST1 and NID2 methylation as a diagnostic test for bladder cancer. Urol Oncol. 2015; 33:387.e1–387.e6. PMID: 26027762.

43. Hayashi M, Bernert H, Kagohara LT, Maldonado L, Brait M, Schoenberg M, et al. Epigenetic inactivation of VGF associated with urothelial cell carcinoma and its potential as a non-invasive biomarker using urine. Oncotarget. 2014; 5:3350–3361. PMID: 24830820.

44. Christoph F, Weikert S, Kempkensteffen C, Krause H, Schostak M, Miller K, et al. Regularly methylated novel pro-apoptotic genes associated with recurrence in transitional cell carcinoma of the bladder. Int J Cancer. 2006; 119:1396–1402. PMID: 16642478.

45. Dominguez G, Carballido J, Silva J, Silva JM, Garcia JM, Menendez J, et al. p14ARF promoter hypermethylation in plasma DNA as an indicator of disease recurrence in bladder cancer patients. Clin Cancer Res. 2002; 8:980–985. PMID: 11948103.
46. Friedrich MG, Chandrasoma S, Siegmund KD, Weisenberger DJ, Cheng JC, Toma MI, et al. Prognostic relevance of methylation markers in patients with non-muscle invasive bladder carcinoma. Eur J Cancer. 2005; 41:2769–2778. PMID: 16242928.

47. Kim WJ, Kim EJ, Jeong P, Quan C, Kim J, Li QL, et al. RUNX3 inactivation by point mutations and aberrant DNA methylation in bladder tumors. Cancer Res. 2005; 65:9347–9354. PMID: 16230397.

48. Tada Y, Wada M, Taguchi K, Mochida Y, Kinugawa N, Tsuneyoshi M, et al. The association of death-associated protein kinase hypermethylation with early recurrence in superficial bladder cancers. Cancer Res. 2002; 62:4048–4053. PMID: 12124340.
49. Kim EJ, Kim YJ, Jeong P, Ha YS, Bae SC, Kim WJ. Methylation of the RUNX3 promoter as a potential prognostic marker for bladder tumor. J Urol. 2008; 180:1141–1145. PMID: 18639281.

50. Maruyama R, Toyooka S, Toyooka KO, Harada K, Virmani AK, Zochbauer-Muller S, et al. Aberrant promoter methylation profile of bladder cancer and its relationship to clinicopathological features. Cancer Res. 2001; 61:8659–8663. PMID: 11751381.
51. Catto JW, Azzouzi AR, Rehman I, Feeley KM, Cross SS, Amira N, et al. Promoter hypermethylation is associated with tumor location, stage, and subsequent progression in transitional cell carcinoma. J Clin Oncol. 2005; 23:2903–2910. PMID: 15753461.

52. Marsit CJ, Karagas MR, Andrew A, Liu M, Danaee H, Schned AR, et al. Epigenetic inactivation of SFRP genes and TP53 alteration act jointly as markers of invasive bladder cancer. Cancer Res. 2005; 65:7081–7085. PMID: 16103055.

53. Kawamoto K, Enokida H, Gotanda T, Kubo H, Nishiyama K, Kawahara M, et al. p16INK4a and p14ARF methylation as a potential biomarker for human bladder cancer. Biochem Biophys Res Commun. 2006; 339:790–796. PMID: 16316628.

54. Yates DR, Rehman I, Abbod MF, Meuth M, Cross SS, Linkens DA, et al. Promoter hypermethylation identifies progression risk in bladder cancer. Clin Cancer Res. 2007; 13:2046–2053. PMID: 17404085.

55. Aleman A, Adrien L, Lopez-Serra L, Cordon-Cardo C, Esteller M, Belbin TJ, et al. Identification of DNA hypermethylation of SOX9 in association with bladder cancer progression using CpG microarrays. Br J Cancer. 2008; 98:466–473. PMID: 18087279.

56. Ellinger J, El Kassem N, Heukamp LC, Matthews S, Cubukluoz F, Kahl P, et al. Hypermethylation of cell-free serum DNA indicates worse outcome in patients with bladder cancer. J Urol. 2008; 179:346–352. PMID: 18006010.

57. Alvarez-Múgica M, Cebrian V, Fernandez-Gomez JM, Fresno F, Escaf S, Sanchez-Carbayo M. Myopodin methylation is associated with clinical outcome in patients with T1G3 bladder cancer. J Urol. 2010; 184:1507–1513. PMID: 20723929.

58. Agundez M, Grau L, Palou J, Algaba F, Villavicencio H, Sanchez-Carbayo M. Evaluation of the methylation status of tumour suppressor genes for predicting bacillus Calmette-Guérin response in patients with T1G3 high-risk bladder tumours. Eur Urol. 2011; 60:131–140. PMID: 21514719.

59. Cebrian V, Fierro M, Orenes-Pinero E, Grau L, Moya P, Ecke T, et al. KISS1 methylation and expression as tumor stratification biomarkers and clinical outcome prognosticators for bladder cancer patients. Am J Pathol. 2011; 179:540–546. PMID: 21683672.

60. Kandimalla R, van Tilborg AA, Kompier LC, Stumpel DJ, Stam RW, Bangma CH, et al. Genome-wide analysis of CpG island methylation in bladder cancer identified TBX2, TBX3, GATA2, and ZIC4 as pTa-specific prognostic markers. Eur Urol. 2012; 61:1245–1256. PMID: 22284968.

61. Lin YL, Liu XQ, Li WP, Sun G, Zhang CT. Promoter methylation of H-cadherin is a potential biomarker in patients with bladder transitional cell carcinoma. Int Urol Nephrol. 2012; 44:111–117. PMID: 21516472.

62. Lin HH, Ke HL, Wu WJ, Lee YH, Chang LL. Hypermethylation of E-cadherin, p16, p14, and RASSF1A genes in pathologically normal urothelium predict bladder recurrence of bladder cancer after transurethral resection. Urol Oncol. 2012; 30:177–181. PMID: 20800513.

63. Kim YJ, Yoon HY, Kim JS, Kang HW, Min BD, Kim SK, et al. HOXA9, ISL1 and ALDH1A3 methylation patterns as prognostic markers for nonmuscle invasive bladder cancer: arraybased DNA methylation and expression profiling. Int J Cancer. 2013; 133:1135–1142. PMID: 23436614.
64. Alvarez-Mugica M, Fernandez-Gomez JM, Cebrian V, Fresno F, Escaf S, Sanchez-Carbayo M. Polyamine-modulated factor-1 methylation predicts Bacillus Calmette-Guerin response in patients with high-grade non-muscle-invasive bladder carcinoma. Eur Urol. 2013; 63:364–370. PMID: 22682992.
65. García-Baquero R, Puerta P, Beltran M, Alvarez-Mujica M, Alvarez-Ossorio JL, Sanchez-Carbayo M. Methylation of tumor suppressor genes in a novel panel predicts clinical outcome in paraffin-embedded bladder tumors. Tumour Biol. 2014; 35:5777–5786. PMID: 24577895.

66. Li H, Wang J, Xiao W, Xia D, Lang B, Wang T, et al. Epigenetic inactivation of KLF4 is associated with urothelial cancer progression and early recurrence. J Urol. 2014; 191:493–501. PMID: 24018236.

67. Lin YL, Wang YL, Ma JG, Li WP. Clinical significance of protocadherin 8 (PCDH8) promoter methylation in non-muscle invasive bladder cancer. J Exp Clin Cancer Res. 2014; 33:68. PMID: 25927589.

68. Sacristan R, Gonzalez C, Fernandez-Gomez JM, Fresno F, Escaf S, Sanchez-Carbayo M. Molecular classification of nonmuscle-invasive bladder cancer (pTa low-grade, pT1 lowg-rade, and pT1 high-grade subgroups) using methylation of tumor-suppressor genes. J Mol Diagn. 2014; 16:564–572. PMID: 24998186.

69. Wang XB, Lin YL, Li ZG, Ma JH, Li J, Ma JG. Protocadherin 17 promoter methylation in tumour tissue from patients with bladder transitional cell carcinoma. J Int Med Res. 2014; 42:292–299. PMID: 24567353.

70. Beukers W, Kandimalla R, Masius RG, Vermeij M, Kranse R, van Leenders GJ, et al. Stratification based on methylation of TBX2 and TBX3 into three molecular grades predicts progression in patients with pTa-bladder cancer. Mod Pathol. 2015; 28:515–522. PMID: 25394776.

71. Kim YW, Yoon HY, Seo SP, Lee SK, Kang HW, Kim WT, et al. Clinical implications and prognostic values of prostate cancer susceptibility candidate methylation in primary nonmuscle invasive bladder cancer. Dis Markers. 2015; 2015:402963. PMID: 26074659.
72. Ellinger J, Muller SC, Dietrich D. Epigenetic biomarkers in the blood of patients with urological malignancies. Expert Rev Mol Diagn. 2015; 15:505–516. PMID: 25719388.

73. Valenzuela MT, Galisteo R, Zuluaga A, Villalobos M, Nunez MI, Oliver FJ, et al. Assessing the use of p16(INK4a) promoter gene methylation in serum for detection of bladder cancer. Eur Urol. 2002; 42:622–628. PMID: 12477660.

74. Jablonowski Z, Reszka E, Gromadzinska J, Wasowicz W, Sosnowski M. Hypermethylation of p16 and DAPK promoter gene regions in patients with non-invasive urinary bladder cancer. Arch Med Sci. 2011; 7:512–516. PMID: 22295037.
75. Hauser S, Kogej M, Fechner G, Von Pezold J, Vorreuther R, Lummen G, et al. Serum DNA hypermethylation in patients with bladder cancer: results of a prospective multicenter study. Anticancer Res. 2013; 33:779–784. PMID: 23482744.
76. Lin YL, Sun G, Liu XQ, Li WP, Ma JG. Clinical significance of CDH13 promoter methylation in serum samples from patients with bladder transitional cell carcinoma. J Int Med Res. 2011; 39:179–186. PMID: 21672320.

77. Lin YL, Li ZG, He ZK, Guan TY, Ma JG. Clinical and prognostic significance of protocadherin-10 (PCDH10) promoter methylation in bladder cancer. J Int Med Res. 2012; 40:2117–2123. PMID: 23321168.

78. Luo ZG, Li ZG, Gui SL, Chi BJ, Ma JG. Protocadherin-17 promoter methylation in serum-derived DNA is associated with poor prognosis of bladder cancer. J Int Med Res. 2014; 42:35–41. PMID: 24366498.

Table 1
Useful combinations of urinary methylation markers for bladder cancer diagnosis
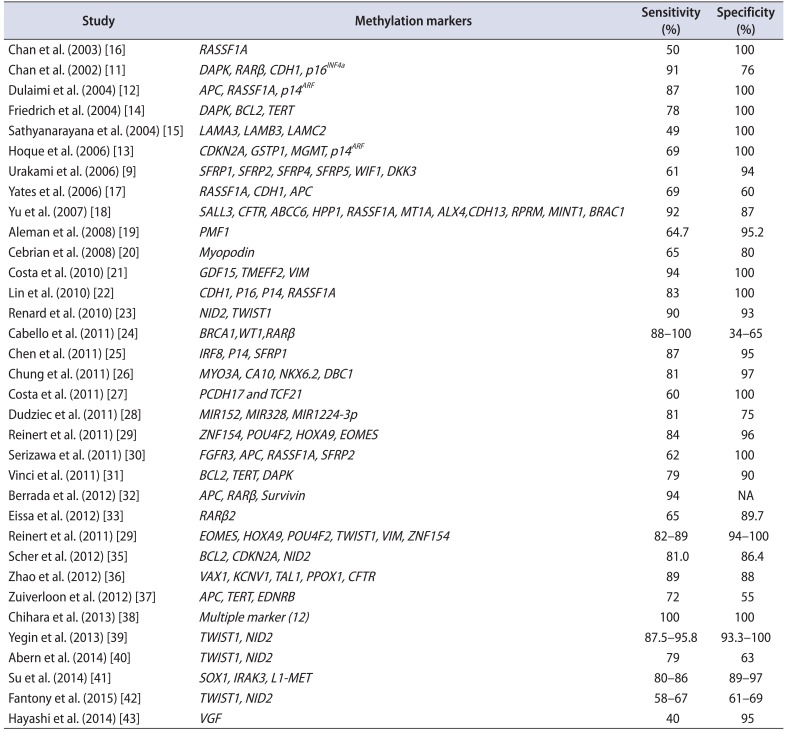
| Study | Methylation markers | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| Chan et al. (2003) [16] | RASSF1A | 50 | 100 |
| Chan et al. (2002) [11] | DAPK, RARβ, CDH1, p16INF4a | 91 | 76 |
| Dulaimi et al. (2004) [12] | APC, RASSF1A, p14ARF | 87 | 100 |
| Friedrich et al. (2004) [14] | DAPK, BCL2, TERT | 78 | 100 |
| Sathyanarayana et al. (2004) [15] | LAMA3, LAMB3, LAMC2 | 49 | 100 |
| Hoque et al. (2006) [13] | CDKN2A, GSTP1, MGMT, p14ARF | 69 | 100 |
| Urakami et al. (2006) [9] | SFRP1, SFRP2, SFRP4, SFRP5, WIF1, DKK3 | 61 | 94 |
| Yates et al. (2006) [17] | RASSF1A, CDH1, APC | 69 | 60 |
| Yu et al. (2007) [18] | SALL3, CFTR, ABCC6, HPP1, RASSF1A, MT1A, ALX4,CDH13, RPRM, MINT1, BRAC1 | 92 | 87 |
| Aleman et al. (2008) [19] | PMF1 | 64.7 | 95.2 |
| Cebrian et al. (2008) [20] | Myopodin | 65 | 80 |
| Costa et al. (2010) [21] | GDF15, TMEFF2, VIM | 94 | 100 |
| Lin et al. (2010) [22] | CDH1, P16, P14, RASSF1A | 83 | 100 |
| Renard et al. (2010) [23] | NID2, TWIST1 | 90 | 93 |
| Cabello et al. (2011) [24] | BRCA1,WT1,RARβ | 88–100 | 34–65 |
| Chen et al. (2011) [25] | IRF8, P14, SFRP1 | 87 | 95 |
| Chung et al. (2011) [26] | MYO3A, CA10, NKX6.2, DBC1 | 81 | 97 |
| Costa et al. (2011) [27] | PCDH17 and TCF21 | 60 | 100 |
| Dudziec et al. (2011) [28] | MIR152, MIR328, MIR1224-3p | 81 | 75 |
| Reinert et al. (2011) [29] | ZNF154, POU4F2, HOXA9, EOMES | 84 | 96 |
| Serizawa et al. (2011) [30] | FGFR3, APC, RASSF1A, SFRP2 | 62 | 100 |
| Vinci et al. (2011) [31] | BCL2, TERT, DAPK | 79 | 90 |
| Berrada et al. (2012) [32] | APC, RARβ, Survivin | 94 | NA |
| Eissa et al. (2012) [33] | RARβ2 | 65 | 89.7 |
| Reinert et al. (2011) [29] | EOMES, HOXA9, POU4F2, TWIST1, VIM, ZNF154 | 82–89 | 94–100 |
| Scher et al. (2012) [35] | BCL2, CDKN2A, NID2 | 81 | 86.4 |
| Zhao et al. (2012) [36] | VAX1, KCNV1, TAL1, PPOX1, CFTR | 89 | 88 |
| Zuiverloon et al. (2012) [37] | APC, TERT, EDNRB | 72 | 55 |
| Chihara et al. (2013) [38] | Multiple marker (12) | 100 | 100 |
| Yegin et al. (2013) [39] | TWIST1, NID2 | 87.5–95.8 | 93.3–100 |
| Abern et al. (2014) [40] | TWIST1, NID2 | 79 | 63 |
| Su et al. (2014) [41] | SOX1, IRAK3, L1-MET | 80–86 | 89–97 |
| Fantony et al. (2015) [42] | TWIST1, NID2 | 58–67 | 61–69 |
| Hayashi et al. (2014) [43] | VGF | 40 | 95 |
Table 2
Predictive value of DNA methylation status for bladder cancer prognosis
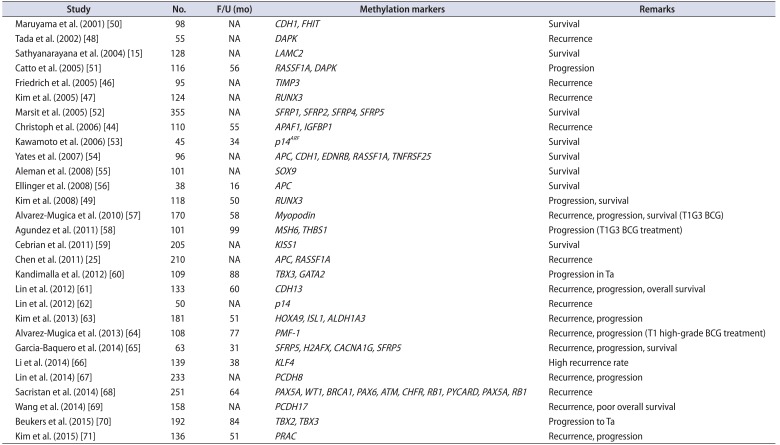
| Study | No. | F/U (mo) | Methylation markers | Remarks |
|---|---|---|---|---|
| Maruyama et al. (2001) [50] | 98 | NA | CDH1, FHIT | Survival |
| Tada et al. (2002) [48] | 55 | NA | DAPK | Recurrence |
| Sathyanarayana et al. (2004) [15] | 128 | NA | LAMC2 | Survival |
| Catto et al. (2005) [51] | 116 | 56 | RASSF1A, DAPK | Progression |
| Friedrich et al. (2005) [46] | 95 | NA | TIMP3 | Recurrence |
| Kim et al. (2005) [47] | 124 | NA | RUNX3 | Recurrence |
| Marsit et al. (2005) [52] | 355 | NA | SFRP1, SFRP2, SFRP4, SFRP5 | Survival |
| Christoph et al. (2006) [44] | 110 | 55 | APAF1, IGFBP1 | Recurrence |
| Kawamoto et al. (2006) [53] | 45 | 34 | p14ARF | Survival |
| Yates et al. (2007) [54] | 96 | NA | APC, CDH1, EDNRB, RASSF1A, TNFRSF25 | Survival |
| Aleman et al. (2008) [55] | 101 | NA | SOX9 | Survival |
| Ellinger et al. (2008) [56] | 38 | 16 | APC | Survival |
| Kim et al. (2008) [49] | 118 | 50 | RUNX3 | Progression, survival |
| Alvarez-Mugica et al. (2010) [57] | 170 | 58 | Myopodin | Recurrence, progression, survival (T1G3 BCG) |
| Agundez et al. (2011) [58] | 101 | 99 | MSH6, THBS1 | Progression (T1G3 BCG treatment) |
| Cebrian et al. (2011) [59] | 205 | NA | KISS1 | Survival |
| Chen et al. (2011) [25] | 210 | NA | APC, RASSF1A | Recurrence |
| Kandimalla et al. (2012) [60] | 109 | 88 | TBX3, GATA2 | Progression in Ta |
| Lin et al. (2012) [61] | 133 | 60 | CDH13 | Recurrence, progression, overall survival |
| Lin et al. (2012) [62] | 50 | NA | p14 | Recurrence |
| Kim et al. (2013) [63] | 181 | 51 | HOXA9, ISL1, ALDH1A3 | Recurrence, progression |
| Alvarez-Mugica et al. (2013) [64] | 108 | 77 | PMF-1 | Recurrence, progression (T1 high-grade BCG treatment) |
| Garcia-Baquero et al. (2014) [65] | 63 | 31 | SFRP5, H2AFX, CACNA1G, SFRP5 | Recurrence, progression, survival |
| Li et al. (2014) [66] | 139 | 38 | KLF4 | High recurrence rate |
| Lin et al. (2014) [67] | 233 | NA | PCDH8 | Recurrence, progression |
| Sacristan et al. (2014) [68] | 251 | 64 | PAX5A, WT1, BRCA1, PAX6, ATM, CHFR, RB1, PYCARD, PAX5A, RB1 | Recurrence |
| Wang et al. (2014) [69] | 158 | NA | PCDH17 | Recurrence, poor overall survival |
| Beukers et al. (2015) [70] | 192 | 84 | TBX2, TBX3 | Progression to Ta |
| Kim et al. (2015) [71] | 136 | 51 | PRAC | Recurrence, progression |
Table 3
Blood-based methylation markers in bladder cancer
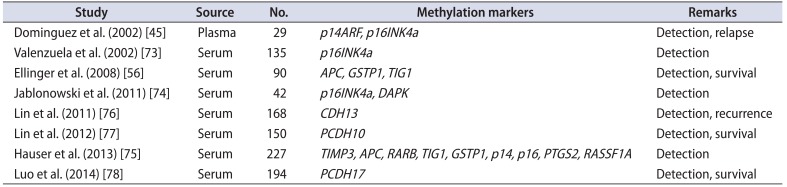
| Study | Source | No. | Methylation markers | Remarks |
|---|---|---|---|---|
| Dominguez et al. (2002) [45] | Plasma | 29 | p14ARF, p16INK4a | Detection, relapse |
| Valenzuela et al. (2002) [73] | Serum | 135 | p16INK4a | Detection |
| Ellinger et al. (2008) [56] | Serum | 90 | APC, GSTP1, TIG1 | Detection, survival |
| Jablonowski et al. (2011) [74] | Serum | 42 | p16INK4a, DAPK | Detection |
| Lin et al. (2011) [76] | Serum | 168 | CDH13 | Detection, recurrence |
| Lin et al. (2012) [77] | Serum | 150 | PCDH10 | Detection, survival |
| Hauser et al. (2013) [75] | Serum | 227 | TIMP3, APC, RARB, TIG1, GSTP1, p14, p16, PTGS2, RASSF1A | Detection |
| Luo et al. (2014) [78] | Serum | 194 | PCDH17 | Detection, survival |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download