Abstract
As the population ages and life expectancy increases in the human population, more individuals will be diagnosed with bladder cancer (BC). The definition of who is elderly is likely to change in the future from the commonly used cut-off of ≥75 years of age. Physiological rather than chronological age is key. BC care in the elderly is likely to become a very common problem in daily practice. Concerns have been raised that senior BC patients are not given treatments that could cure their disease. Clinicians lack quantitative and reliable estimates of competing mortality risks when considering treatments for BC. Majority of patients diagnosed with BC are elderly, making treatment decisions complex with their increasing number of comorbidities. A multidisciplinary approach to these patients may be a way to incorporate discussion from various disciplines regarding treatment options available. Here we review various treatment options for elderly patients with muscle invasive BC and nonmuscle invasive BC. We include differences in treatments from robotic versus open radical cystectomy, various urinary diversion techniques, chemotherapy, radiation therapy and combination treatments. In clinical practice, treatment decisions for elderly patients should be done on a case-by-case basis, tailored to each patient with their specific histories and comorbidities considered. Some healthy elderly patients may be better candidates for extensive curative treatments than their younger counterparts. This implies that these important, life-altering decisions cannot be solely based on age as many other factors can affect patient survival outcomes.
Bladder Cancer (BC) is the 5th most common cancer with its incidence increasing with age (median age at diagnosis of 73 years). Individuals aged 75–84 years account for the largest percentage, at 30%, of new cases [123]. Data collected by the National Cancer Institute (Surveillance, Epidemiology, and End Results [SEER] Program), the Centers for Disease Control and Prevention (National Program of Cancer Registries), and the North American Association of Central Cancer Registries predicts 76,960 new urinary BC cases in 2016 with an estimated 18,000 deaths in the United States alone [4]. This is an estimated increase of approximately 1,000 new cases and 2,000 deaths from 2015 [5]. In Canada, 2015 statistics estimated that 8,300 Canadians will be diagnosed with BC with 2,300 dying from the disease [3].
Population aging is a global phenomenon with significant implications for health care systems in the developed world, especially with respect to cancer care. With a dramatic change in the age pyramid in Western countries, and a number of octogenarians (aged ≥80 years) expected to increase sharply, BC care in the elderly is likely to become a very common problem in daily practice.
In 2012, over 5.2 million people in Canada were over 65 years of age, representing 14.9% of the total population [6]. Of these, approximately 1.4 million (4.2% of the total population) were octogenarians and this number is expected to increase exponentially over the next decades. According to the projection scenarios, the population aged 80 and over would increase from 1.4 million in 2013 to between 4.0 million and 4.9 million by 2045, representing about 10% of the total Canadian population [7].
The number of centenarians in Canada (persons aged ≥100 years) is expected to multiply nine times over the next 50 years. Even the definition of elderly patients is likely to become a moving target in the future! Although advanced age may be associated with worse outcomes in BC, stage and grade at diagnosis remain key determinants of prognosis. However the elephant in the room might also be the whopping number of elderly patients with BC who are not treated at all.
Concerns have been raised that older BC patients are not given treatments that could cure their disease. Clinicians lack quantitative and reliable estimates of competing mortality risks when considering treatments for BC. "Guesstimating" these competing risks, rather than objectively quantifying them is a common limitation.
While more than half of patients under the age of 60 had potentially curative treatments such as surgery or radiotherapy, a third of patients in their 70s and only 12% of patients over 80 were given such procedures. This was according to a study which examined the records of 3,300 BC patients diagnosed in Sheffield, United Kingdom, between 1994 and 2009 [8]. These authors also found that patients over 70 were more likely to die of the disease than younger patients. Elderly patients had indeed a higher proportion of more aggressive tumors and were less likely to receive radical treatments such as radiotherapy or surgery. From this study, it appeared that not enough older patients are being offered treatments that could increase their chance of survival [8].
Decisions to treat or not are never easy and need to be balanced with quality of life (QoL), but it appears as of utmost importance for patients of all ages to be given the option of a possible cure when it is still feasible.
The situation in North America is disturbingly similar. Data from the SEER in the United States on the treatment of muscle invasive bladder cancer (MIBC) showed that a staggering 51% of patients did not receive any definitive therapy at all. It is certainly possible that many of these elderly patients may have been eligible for some form of therapy other than cystectomy like radiation therapy, if they were poor candidates for cystectomy [9].
Although studies have found that majority of patients diagnosed with BC are elderly [210], who is elderly is not agreed upon. Previously, patients who were ≥65 years were generally considered to be part of this population but this limit is constantly pushed upwards [11]. It is not uncommon to discuss in Toronto the postoperative course after radical cystectomy (RadC) with patients who are ≥80 years of age (looking at least 10 years younger than their stated age) whose only concern is when they will be able to resume golfing or skiing. Twenty years ago this might have looked like a joke but nowadays this is a legitimate concern in an age group who is constantly challenging and pushing further the definition of 'elderly'.
Although the term 'elderly' refers to advanced chronological age, perhaps more important factors in determining treatment decisions are the functional status and associated comorbidities of the individual patient. "Fit" elderly patients, no matter what age, should be considered for aggressive interventions for BC, while those with significant health issues may not benefit. Accurately defining the latter group is challenging and error-prone. Patients who are "fit" have no significant functional impairments and/or comorbidities. Fit patients should receive evidence-based care as often as possible. On the other end of the spectrum, older patients who are "frail" demonstrate dependence in basic functional tasks, significantly impaired mobility, significant comorbidities, and/or at least one significant geriatric syndrome, such as falls. These patients are at high risk for toxicities from cancer treatment. Decision-making is more complex for patients who are vulnerable and have concomitant mild functional or cognitive issues, well-controlled and nonlife threatening comorbid conditions, and/or depression.
One condition often underestimated and overlooked, is depression. We have been struck by the number of elderly patients undergoing cystectomy, smoothly recovering through their surgical postoperative course, but displayed at times severe forms of depression. We believe this aspect is often overlooked and worth additional studies.
Most studies nowadays use 75 years of age to define elderly patients [11]. This population has been associated with many comorbidities and a shorter life expectancy [210]. Comorbiditity and age have been found to be independent predictors of overall survival (OS) in BC patients [121314].
MIBC patients can undergo various treatments such as chemotherapy, surgery, radiation therapy or a combination of treatments. Age of patients can impact which treatment they do receive. As the decision of treatment for elderly MIBC patients is complex, a multidisciplinary approach should be considered. This can involve many different departments such as surgical, medical and radiation oncology, internal medicine, physical therapy and nutrition [1516]. Advantages of a multidisciplinary bladder cancer clinic (MDBCC) include providing patients with discussions with a Urologic and Radiation Oncologists in addition to subsequent Medical Oncologist evaluation (if necessary) to make an appropriate treatment decision. Additional investigations can also be made at his time [16]. The MDBCC was started in 2008 in Toronto with the aim to deal with complex BC cases in order to offer and discuss all available treatment options to patients including surgery or trimodality (radiotherapy, chemotherapy, and transurethral resection [TUR]). Hermanns et al. [16] found that following additional investigations in a MDBCC, 42% of patients had a change in stage and 29% had a change in treatment. While some patients did undergo RadC, others were offered a bladder preservation approach (trimodal therapy with chemoradiation). Using this team approach to address these complex BC cases, patients are more likely to be treated and less likely to fall through the cracks, ultimately improving care.
With life expectancy increasing in the human population, it is important to ensure patients of all ages receive proper treatment for their disease and are not turned away due to age.
Older patients have been found to have higher stage at diagnosis, higher rate of upstaging on final pathology and a longer delay to definitive therapy [17181920]. When selecting patients to undergo RadC, the institution and urologist (training and experience) are important in the outcome of the procedure [21]. Elderly patients undergo RadC for MIBC less often than younger patients [9132223]. Specifically using the SEER-Medicare data, two separate studies showed that older patients (age >80 [24], >75 [25]) are less likely treated with RadC. Large studies have also showed age to be associated with complications after undergoing RadC [2627].
RadC is a complex surgical procedure, associated with complications, morbidity and extended hospital stays, even in experienced hands [2829]. Patients ≥75 years often have longer hospital stays (5 days vs. 4 days, p=0.03) and higher minor complication rate (72% vs. 51%, p=0.04) than younger patients [30].
Age has not been found to be a surrogate for comorbidity (r=0.151 cutoff r>0.5 for interaction, spearman rank correlation coefficient). Interesting to note, in one study, age was a factor for OS (p=0.0001) in BC patients however, in a subgroup of those patients treated by cystectomy, age was not significant upon multivariate analysis (hazard ratio, 1.19; 95% confidence interval, 0.92–1.54; p=0.1810) [12]. Another study found that older patients had a lower OS possibly brought on by a higher proportion of severe comorbidities (p<0.01) compared to younger BC patients [13].
Horovitz et al. [31] in Toronto analyzed the impact of a patient's age following RadC by subdividing 605 patients into 4 age groups (≤59, 60–69, 70–79, ≥80). Results of this study showed that the rate of neobladder and continent cutaneous diversion use decreased with increasing age.
When deciding on the type of urinary diversion, renal function is an important factor to consider [32]. With a glomerular filtration rate (GFR) >50 mL/min, the capacity to avoid metabolic acidosis or other consequences can be avoided. GFR however does decrease in age (approximately 1 mL per year after 40 years of age) as does renal blood flow (1% per year after 50 years of age) [33]. Elderly patients may not be able to preserve homeostasis after a continent diversion, thus it is important the appropriate patients are selected for this. Siddiqui and Izawa [32] reported that the diversion of choice, for elderly patients and those with reduced renal function following RadC, is an Ileal conduit (IC). IC has been found to pose the least metabolic changes with excellent QoL reported by patients.
Studies have shown that the use of adjuvant treatments differs in age groups (15%, ≤79 years vs. 2%, ≥80 years p=0.04). No differences were found in gender, clinical stage, treatment delays from the time of last TUR to RadC or rates of neoadjuvant treatment among various age groups [31]. Studies have shown that when patients undergoing the same procedure (i.e., RadC), recurrence free survival (RFS), disease specific survival (DSS) and OS are similar among patients of all age groups [173134].
In a series from Memorial Sloan Kettering studying cystectomy in octogenarians, RadC in older patients with BC provided similar disease control and survival outcomes with risks of high grade perioperative morbidity comparable to those in younger patients [35], supporting that chronological age per se is not a factor to exclude patients from a potential curative therapy.
The risk of perioperative mortality does not seem to be affected by the type of urinary diversion used. When patients were asked to report their QoL following their surgical procedure (neobladder or IC), they generally reported high scores with no statistical differences between the two groups [36].
Skinner et al. [37] noted that age alone should not be the sole decider for patients with MIBC to undergo cystectomy. As a rule of thumb, it was suggested that curative therapy should be offered if the patient has an estimated life expectancy of at least 2 years with no morbidities making surgical intervention unlikely to be successful.
Froehner et al. [11] described an inverse relationship between age and continence recovery following orthotropic bladder substitution, and potency recovery as one would have expected. They also found that blood loss, transfusion rates and cardiovascular morbidity occur more often in older patients.
Age has been found to be a predictive factor for 90-date mortality following RadC in a multinational study from 18 European centres [38]. Other studies have also reported 90-day mortality rates increase with age; 5.5% in patients ≥65 years [39], 6.2%–7.5% in those ≥75 years [1840], and 10%–11% in those ≥80 years [4142].
In a recent study of 5,207 MIBC patients who underwent RadC the 30- and 90-day mortality rates were reported as 5.2% and 10.6%, respectively. When broken down by age, 90-day mortality rates were higher than those previously reported; 65–69, 70–79, and >80 years were 6.4%, 10.1%, and 14.8% (p<0.001) respectively. The 90-day mortality rates also increased with increasing Charlson comorbidity index (CCI, 0, 1, 2, and 3): 6.3, 10.3, 12.6, and 15.9% p<0.001) [43].
Reporting of complications following cystectomy has been found to be heterogeneous, although frequency and spectrum of complications in the elderly vary widely amongst different studies [11]. Disorientation or delirium was found to occur in 5%–20% of patients in six studies reviewed by Froehner et al. [11] however many studies have not reported these events.
Clark et al. [34] did not find any difference in mortality and early diversion-related complication rates in the elderly who underwent RadC treatment for the MIBC. A retrospective study on patients receiving IC or orthotopic neobladder following RadC showed similar results [3644]. The most commonly used urinary diversion following RadC remained to be IC (~70%) in the elderly population [11].
Another alternative to an IC, in very high risk elderly patients, that can be performed quickly with few early and late postoperative complications is a cutaneous ureterostomy [45]. In a recent review 21 studies compared at least one QoL metric between patients who received an IC or neobladder following RadC. Sixteen studies reported no differences in QoL, Four reported improved QoL with a neobladder, and 1 reported better QoL with a conduit [46]. Another study reported no difference between patients who received either an IC or neobladder [36]. A study from 2012 found that patients who underwent IC had higher health-related QoL compared to those who underwent neobladder. The authors reported that it may have been due to the postoperative expectations of patients with a neobladder may have been a disappointment, decreasing their QoL [47].
Many studies have recommended that RadC should be offered to appropriately selected older patients who have advanced disease [1731]. Orthotopic neobladder could also be considered for well-selected elderly patients who undergo RadC but caution should prevail [364448]. One study (n=224 patients >75 years) found that overall and cancer specific survival for orthotopic neobladder, IC and urterocutaneostomy were 90 and 98, 47 and 91, and 11 and 12 months, respectively. Although these results point to patients undergoing orthotopic neobladder doing better than their counterparts, they had significantly lower pT stages [48]. Longo et al. [49] reported that cutaneous ureterostomy is a valid alternative to IC in elderly patients with comorbidities or perioperative complications, without significant impairment to their QoL. Patients undergoing IC had longer operating times, higher estimated blood loss, need for intensive care monitoring, time to drain removal and length of hospital stay. Fig. 1 shows the 90 day incidence of adverse events by organ system.
Continence rates were found to be lower in younger patients with an ileal neobladder. One study reported in selected patients ≥65 had high continence rates (>90% daytime, 80% nighttime – complete dryness or loss of no more than a few drops 1–2 times/mo) while another reported less favourable rates in patients ≥75 years (56% daytime and 25% nighttime continence and 30% patients requiring intermittent catheterization due to chronic retention) [11]. 5-year survival rates following RadC, regardless of age was reported to be between 45%–60% [11].
A publically available universal surgical risk calculator (http://riskcalculator.facs.org/) was developed using standardized clinical data from the American College of Surgeons National Surgical Quality Improvement Program. 21 preoperative factors are used to estimate postoperative mortality, morbidity, and specific complications with excellent performance [50]. This tool may assist physicians in their management of patients with MIBC.
Another tool that may aid in determining if an elderly patient is fit for a complicated surgical procedure is for them to complete a Comprehensive Geriatric Assessment (CGA). The CGA assesses many areas of health including function, comorbidities, socioeconomics, cognition, emotion, medications, nutrition, dementia, fall risk, etc. [51]. This tool has been utilized and validated showing its effectiveness in identifying the true health status of these patients [52]. Its use in surgical oncology, specifically prior to RadC, is still being investigated [5354].
QoL is a critical issue in elderly patients with cancer. In the geriatric population, cancer is often associated with other chronic conditions possibly affecting QoL and the CGA seems to help in this setting. A prospective study in France aimed to evaluate the validity of two QoL questionnaires in older cancer patients (median age, 76 years) found that cognitive function and functional status, two factors likely to influence the value of QoL self-assessment, were poorly taken into account whereas they were correctly explored by the CGA [55]. Noteworthy the approach to elderly cancer patients' management varies across the cultures and this should be recognized [5657].
Lots of studies are now comparing minimally invasive approaches for RadC versus open surgery. Xia et al. [58] reviewed 19 studies, 787 patients underwent Robot Assisted RadC (RARC) while 992 underwent Open RadC (ORC). They found that RARC patients had lower overall perioperative complication rates within 30 days and 90 days (p=0.005 and p=0.0002, respectively). These patients also had more lymph node yields (p=0.009), less estimated blood loss (p<0.00001), lower need for perioperative and intraoperative transfusions (p<0.0001 and p<0.0001, respectively), and shorter postoperative length of stay (p=0.0002) than ORC patients. Similar results have been reported in many studies [59606162636465]. A study of RARC reported 2-year RFS (73%), DSS (74%) and OS (61%) rates in patients from 80–94 years of age (median, 83 years; 68% ≥pT2) [66]. However this enthusiasm for RARC is not shared by all and randomized studies have yielded conflicting results.
A randomized trial at Memorial Sloane Kettering failed to identify a large advantage for robot-assisted techniques over standard open surgery for patients undergoing RadC/pelvic lymph node dissection (PLND) and urinary diversion. Similar 90-day complication rates, hospital stay, pathologic outcomes, and 3- and 6-month QoL outcomes were observed regardless of surgical technique [6768].
As elderly patients potentially experience a higher number of complications following RadC minimizing complications is key in this age group. It remains to be proven whether RARC may be better suited for elderly patients. The RAZOR (randomized open vs robotic cystectomy) study will compare ORC versus RARC, PLND and urinary diversion for oncological outcomes, complications and health-related QoL measures with a primary endpoint of 2-year progression-free survival (PFS). RAZOR is a multi-institutional, randomized, noninferior, phase III trial that will enroll at least 320 patients with T1–T4, N0–N1, M0 BC with ≈160 patients in both the RARC and ORC arms at 15 participating institutions. Full data from the RAZOR trial are not expected until 2017 [69].
As mentioned previously, patients with MIBC can undergo a variety and combinations of treatments. Curative treatments for patients with MIBC have been reported to be either RadC or trimodal therapy [29]. Poor surgical candidates or those who want to avoid a major surgery can choose to undergo a combined modality treatment, with RadC saved for treatment failure. This is something that is frequently discussed through MDBCC.
For instance in Toronto's MDBCC, a team of specialized oncologists can provide a thorough discussion with patients as to what the best care for them can be [16]. Through the MDBCC, MIBC patients can decide undergo trimodal bladder preservation treatments, with RadC as salvage therapy [70]. Trimodal therapy in elderly patients has been found to be well tolerated with outcomes similar to that of RadC [71]. Outcomes of trimodal therapy in patients ≥75 years have been found to be comparable to those of younger patients [7273].
Nayan et al. [70] found that over time, more patients at the MDBCC underwent neoadjuvant chemotherapy and fewer patients underwent adjuvant chemotherapy. A systemic review found an association between patient survival and multidisciplinary care [74]. Chemotherapy use and toxicities in this population has not been vastly studied in this group of patients as most trials have been on younger, healthier patients [15]. One clinical trial showed no significant differences in response rate, survival or toxicities in patients ≥70 years when compared to their younger counterparts treated with chemotherapy for MIBC [75]. A prospective trial on elderly patients (≥75 years) treated with selective bladder preservation showed 33% of patients achieved complete response and 60% carrying on to a full course of combined modality therapy. Five- (60%) and 10-year DSS rates (56%) were similar to that of patients undergoing RadC [76]. Radiation alone can be used to palliate local disease-associated symptoms such as hematuria, dysuria or frequency [2977].
Although various treatments can be offered to patients, beyond RadC, these important decisions should always be discussed with the patient and chosen based on their individual cases.
Relatively little has been published about the efficacy of current therapies in treating nonmuscle invasive bladder cancer (NMIBC) in elderly patients. A multicenter trial found that overall response to either bacillus Calmette-Guérin (BCG) or BCG plus interferon a was decreased in participants ≥80 years of age compared with younger patients [78]. At 24-month follow-up, absolute response was reduced by 22% in patients ≥80 years compared with patients aged 61–70 years (39% vs. 61%). Age remained a significant variable for decreased response to therapy after controlling for multiple other relevant variables.
Herr reported his 20 years' experience at a Memorial Sloan Kettering [79]. Outcome measures included initial response to BCG and tumor-free recurrence. When the series was stratified by age (> or <70 years), a small but significant difference was seen in tumor-free recurrence, favoring the younger group. Increased age seemed to confer a less-durable response to BCG, earlier recurrences and a shorter cancerfree survival time. Nonetheless, following multivariable analysis, only tumor stage and grade remained as significant predictors of response to therapy.
Margel et al. [80] studied a cohort of 238 patients in Toronto. The 2-year PFS was 87% among patients <75 years vs 65% in patients >75 years (p<.001). An age-dependent trend was noted when analyzed by 10-year increment (logrank for trend p=0.011). On multivariable analysis, age was an independent risk factor for progression but recurrence-free survival was similar among age strata.
In clinical practice, a decision for treatment of the elderly patient must be made on a case-by-case basis. Discussions should be held with the patient on the pros and cons of various treatments with a decision made based on all factors. A multidisciplinary approach may be an alternative in assuring the patient that they are indeed receiving the best care and above all, not denied care when they could receive it. Some healthy elderly patients may be better candidates for extensive curative treatments than their younger counterparts. This implies that these important, life-altering decisions cannot be solely based on age as many other factors can affect patient survival outcomes.
With an ageing population and increasing number of BC patients, optimizing therapy for elderly BC patients may become a crucial issue. Studies focusing on this age group should be encouraged and research intensified.
While numerous studies have tried to evaluate surgery or radiation for elderly patients with BC, many studies have failed to incorporate a component of true functional assessment including psychological evaluation, well-being as well as QoL. Evaluation tools that incorporate comorbidities, disabilities and functional status clearly need to be improved, as chronological age per se is a poor predictor of treatment outcomes.
Finally, the present situation where the older the patients are, the less likely they are to be offered treatments that could cure their BC is clearly unacceptable. We should join forces so that all efforts should be done to increase awareness among patients and physicians regarding available therapeutic options in this age group.
References
1. Surveillance, Epidemiology, and End Research Program. SEER stat fact sheets: bladder cancer [Internet]. Bethesda (MD): National Cancer Institute;2016. cited 2016 May 1. Available from: http://seer.cancer.gov/statfacts/html/urinb.html.
2. Shariat SF, Milowsky M, Droller MJ. Bladder cancer in the elderly. Urol Oncol. 2009; 27:653–667. PMID: 19879476.

3. Canadian Cancer Society's Advisory Committee on Cancer Statistics. Canadian Cancer Statistics 2015. Toronto (ON): Canadian Cancer Society;2015.
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66:7–30. PMID: 26742998.

5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015; 65:5–29. PMID: 25559415.

6. Statistics Canada. Annual demographic estimates: Canada, provinces and territories 2012 [Internet]. Otawa (ON): Statistics Canada;2012. cited 2016 May 1. Available from: www.statcan.gc.ca/pub/91-215-x/91-215-x2012000-eng.pdf.
7. Statistics Canada. Population projections for Canada, provinces and territories (91-520-X) [Internet]. Otawa (ON): Statistics Canada;2016. cited 2016 May 1. Available from: http://www5.statcan.gc.ca/olc-cel/olc.action?objId=91-520-X&objType=2&lang=en&limit=0.
8. Noon AP, Albertsen PC, Thomas F, Rosario DJ, Catto JW. Competing mortality in patients diagnosed with bladder cancer: evidence of undertreatment in the elderly and female patients. Br J Cancer. 2013; 108:1534–1540. PMID: 23481180.

9. Gore JL, Litwin MS, Lai J, Yano EM, Madison R, Setodji C, et al. Use of radical cystectomy for patients with invasive bladder cancer. J Natl Cancer Inst. 2010; 102:802–811. PMID: 20400716.

10. Leveridge MJ, Siemens DR, Mackillop WJ, Peng Y, Tannock IF, Berman DM, et al. Radical cystectomy and adjuvant chemotherapy for bladder cancer in the elderly: a population-based study. Urology. 2015; 85:791–798. PMID: 25661830.

11. Froehner M, Brausi MA, Herr HW, Muto G, Studer UE. Complications following radical cystectomy for bladder cancer in the elderly. Eur Urol. 2009; 56:443–454. PMID: 19481861.

12. Megwalu II, Vlahiotis A, Radwan M, Piccirillo JF, Kibel AS. Prognostic impact of comorbidity in patients with bladder cancer. Eur Urol. 2008; 53:581–589. PMID: 17997024.

13. Ha MS, Chang IH. Significance of age and comorbidity as prognostic indicators for patients with bladder cancer. Asian J Androl. 2010; 12:766–774. PMID: 20676116.

14. Fairey AS, Jacobsen NE, Chetner MP, Mador DR, Metcalfe JB, Moore RB, et al. Associations between comorbidity, and overall survival and bladder cancer specific survival after radical cystectomy: results from the Alberta Urology Institute Radical Cystectomy database. J Urol. 2009; 182:85–92. PMID: 19447413.

15. Grubmueller B, Seitz C, Shariat SF. The treatment of muscleinvasive bladder cancer in geriatric patients. Curr Opin Urol. 2016; 26:160–164. PMID: 26765044.

16. Hermanns T, Wei Y, Bhindi B, Satkunasivam R, Athanasopoulos P, Bostrom PJ, et al. A multidisciplinary bladder cancer clinic delivers personalized care for complex bladder cancer patients. Eur Urol Suppl. 2014; 13:e793.
17. Rink M, Dahlem R, Kluth L, Minner S, Ahyai SA, Eichelberg C, et al. Older patients suffer from adverse histopathological features after radical cystectomy. Int J Urol. 2011; 18:576–584. PMID: 21699582.

18. May M, Fritsche HM, Gilfrich C, Brookman-May S, Burger M, Otto W, et al. Influence of older age on survival after radical cystectomy due to urothelial carcinoma of the bladder: survival analysis of a German multi-centre study after curative treatment of urothelial carcinoma of the bladder. Urologe A. 2011; 50:821–829. PMID: 21340593.
19. Nielsen ME, Shariat SF, Karakiewicz PI, Lotan Y, Rogers CG, Amiel GE, et al. Advanced age is associated with poorer bladder cancer-specific survival in patients treated with radical cystectomy. Eur Urol. 2007; 51:699–706. PMID: 17113703.

20. Fleshner NE, Herr HW, Stewart AK, Murphy GP, Mettlin C, Menck HR. The National Cancer Data Base report on bladder carcinoma. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1996; 78:1505–1513. PMID: 8839558.
21. Bhindi B, Yu J, Kuk C, Sridhar SS, Hamilton RJ, Finelli A, et al. The importance of surgeon characteristics on impacting oncologic outcomes for patients undergoing radical cystectomy. J Urol. 2014; 192:714–719. PMID: 24594406.

22. Schrag D, Mitra N, Xu F, Rabbani F, Bach PB, Herr H, et al. Cystectomy for muscle-invasive bladder cancer: patterns and outcomes of care in the Medicare population. Urology. 2005; 65:1118–1125. PMID: 15922428.

23. Fedeli U, Fedewa SA, Ward EM. Treatment of muscle invasive bladder cancer: evidence from the National Cancer Database, 2003 to 2007. J Urol. 2011; 185:72–78. PMID: 21074192.

24. Chamie K, Hu B, Devere White RW, Ellison LM. Cystectomy in the elderly: does the survival benefit in younger patients translate to the octogenarians? BJU Int. 2008; 102:284–290. PMID: 18410437.

25. Prout GR Jr, Wesley MN, Yancik R, Ries LA, Havlik RJ, Edwards BK. Age and comorbidity impact surgical therapy in older bladder carcinoma patients: a population-based study. Cancer. 2005; 104:1638–1647. PMID: 16130136.
26. Hollenbeck BK, Miller DC, Taub D, Dunn RL, Khuri SF, Henderson WG, et al. Identifying risk factors for potentially avoidable complications following radical cystectomy. J Urol. 2005; 174(4 Pt 1):1231–1237. PMID: 16145376.

27. Konety BR, Dhawan V, Allareddy V, Joslyn SA. Impact of hospital and surgeon volume on in-hospital mortality from radical cystectomy: data from the health care utilization project. J Urol. 2005; 173:1695–1700. PMID: 15821560.

28. Shabsigh A, Korets R, Vora KC, Brooks CM, Cronin AM, Savage C, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. 2009; 55:164–174. PMID: 18675501.

29. Guancial EA, Roussel B, Bergsma DP, Bylund KC, Sahasrabudhe D, Messing E, et al. Bladder cancer in the elderly patient: challenges and solutions. Clin Interv Aging. 2015; 10:939–949. PMID: 26089655.
30. Daneshmand S, Ahmadi H, Schuckman AK, Mitra AP, Cai J, Miranda G, et al. Enhanced recovery protocol after radical cystectomy for bladder cancer. J Urol. 2014; 192:50–55. PMID: 24518775.

31. Horovitz D, Turker P, Bostrom PJ, Mirtti T, Nurmi M, Kuk C, et al. Does patient age affect survival after radical cystectomy? BJU Int. 2012; 110(11 Pt B):E486–E493. PMID: 22551360.

32. Siddiqui KM, Izawa JI. Ileal conduit: standard urinary diversion for elderly patients undergoing radical cystectomy. World J Urol. 2016; 34:19–24. PMID: 26475274.

33. Mühlberg W, Platt D. Age-dependent changes of the kidneys: pharmacological implications. Gerontology. 1999; 45:243–253. PMID: 10460985.

34. Clark PE, Stein JP, Groshen SG, Cai J, Miranda G, Lieskovsky G, et al. Radical cystectomy in the elderly: comparison of clincal outcomes between younger and older patients. Cancer. 2005; 104:36–43. PMID: 15912515.
35. Donat SM, Siegrist T, Cronin A, Savage C, Milowsky MI, Herr HW. Radical cystectomy in octogenarians: does morbidity outweigh the potential survival benefits? J Urol. 2010; 183:2171–2177. PMID: 20399461.
36. Sogni F, Brausi M, Frea B, Martinengo C, Faggiano F, Tizzani A, et al. Morbidity and quality of life in elderly patients receiving ileal conduit or orthotopic neobladder after radical cystectomy for invasive bladder cancer. Urology. 2008; 71:919–923. PMID: 18355900.

37. Skinner EC, Stein JP, Skinner DG. Surgical benchmarks for the treatment of invasive bladder cancer. Urol Oncol. 2007; 25:66–71. PMID: 17208142.

38. Aziz A, May M, Burger M, Palisaar RJ, Trinh QD, Fritsche HM, et al. Prediction of 90-day mortality after radical cystectomy for bladder cancer in a prospective European multicenter cohort. Eur Urol. 2014; 66:156–163. PMID: 24388438.
39. Bostrom PJ, Kossi J, Laato M, Nurmi M. Risk factors for mortality and morbidity related to radical cystectomy. BJU Int. 2009; 103:191–196. PMID: 18671789.
40. Zebic N, Weinknecht S, Kroepfl D. Radical cystectomy in patients aged > or = 75 years: an updated review of patients treated with curative and palliative intent. BJU Int. 2005; 95:1211–1214. PMID: 15892803.
41. Mendiola FP, Zorn KC, Gofrit ON, Mikhail AA, Orvieto MA, Msezane LP, et al. Cystectomy in the ninth decade: operative results and long-term survival outcomes. Can J Urol. 2007; 14:3628–3634. PMID: 17784983.
42. Nielsen ME, Mallin K, Weaver MA, Palis B, Stewart A, Winchester DP, et al. Association of hospital volume with conditional 90-day mortality after cystectomy: an analysis of the National Cancer Data Base. BJU Int. 2014; 114:46–55. PMID: 24219110.

43. Schiffmann J, Gandaglia G, Larcher A, Sun M, Tian Z, Shariat SF, et al. Contemporary 90-day mortality rates after radical cystectomy in the elderly. Eur J Surg Oncol. 2014; 40:1738–1745. PMID: 25454826.

44. Hugen CM, Daneshmand S. Orthotopic urinary diversion in the elderly. World J Urol. 2016; 34:13–18. PMID: 26410825.

45. Deliveliotis C, Papatsoris A, Chrisofos M, Dellis A, Liakouras C, Skolarikos A. Urinary diversion in high-risk elderly patients: modified cutaneous ureterostomy or ileal conduit? Urology. 2005; 66:299–304. PMID: 16040096.

46. Ali AS, Hayes MC, Birch B, Dudderidge T, Somani BK. Health related quality of life (HRQoL) after cystectomy: comparison between orthotopic neobladder and ileal conduit diversion. Eur J Surg Oncol. 2015; 41:295–299. PMID: 24913090.

47. Anderson CB, Feurer ID, Large MC, Steinberg GD, Barocas DA, Cookson MS, et al. Psychometric characteristics of a condition-specific, health-related quality-of-life survey: the FACT-Vanderbilt Cystectomy Index. Urology. 2012; 80:77–83. PMID: 22608798.

48. Wuethrich PY, Vidal A, Burkhard FC. There is a place for radical cystectomy and urinary diversion, including orthotopic bladder substitution, in patients aged 75 and older: Results of a retrospective observational analysis from a high-volume center. Urol Oncol. 2016; 34:58.e19–58.e27. PMID: 26420022.

49. Longo N, Imbimbo C, Fusco F, Ficarra V, Mangiapia F, DiLorenzo G, et al. Complications and quality of life in elderly patients with high comorbidities who underwent cutaneous ureterostomy with single stoma or ileal conduit after radical cystectomy. BJU Int. 2016; 3. 02. [Epub]. DOI: 10.1111/bju.13462.
50. Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013; 217:833–842. PMID: 24055383.
51. Hurria A, Cirrincione CT, Muss HB, Kornblith AB, Barry W, Artz AS, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol. 2011; 29:1290–1296. PMID: 21357782.

52. Isbarn H, Jeldres C, Zini L, Perrotte P, Baillargeon-Gagne S, Capitanio U, et al. A population based assessment of perioperative mortality after cystectomy for bladder cancer. J Urol. 2009; 182:70–77. PMID: 19447427.

53. Feng MA, McMillan DT, Crowell K, Muss H, Nielsen ME, Smith AB. Geriatric assessment in surgical oncology: a systematic review. J Surg Res. 2015; 193:265–272. PMID: 25091339.

54. Galsky MD. How I treat bladder cancer in elderly patients. J Geriatr Oncol. 2015; 6:1–7. PMID: 25482022.

55. Terret C, Perol D, Albrand G, Droz JP. Quality of life in geriatric oncology: an evaluation of standard questionnaires in elderly men with urological malignancies. Crit Rev Oncol Hematol. 2011; 77:201–209. PMID: 20338777.
56. Surbone A, Kagawa-Singer M, Terret C, Baider L. The illness trajectory of elderly cancer patients across cultures: SIOG position paper. Ann Oncol. 2007; 18:633–638. PMID: 17028242.

57. Terret C, Droz JP. Editorial. The perception and dissemination of geriatric oncology. Crit Rev Oncol Hematol. 2010; 75:43–46. PMID: 20537904.
58. Xia L, Wang X, Xu T, Zhang X, Zhu Z, Qin L, et al. Robotic versus open radical cystectomy: an updated systematic review and meta-analysis. PLoS One. 2015; 10:e0121032. PMID: 25825873.

59. Ishii H, Rai BP, Stolzenburg JU, Bose P, Chlosta PL, Somani BK, et al. Robotic or open radical cystectomy, which is safer? A systematic review and meta-analysis of comparative studies. J Endourol. 2014; 28:1215–1223. PMID: 25000311.

60. Lin T, Fan X, Zhang C, Xu K, Liu H, Zhang J, et al. A prospective randomised controlled trial of laparoscopic vs open radical cystectomy for bladder cancer: perioperative and oncologic outcomes with 5-year follow-upT Lin et al. Br J Cancer. 2014; 110:842–849. PMID: 24407192.

61. Musch M, Janowski M, Steves A, Roggenbuck U, Boergers A, Davoudi Y, et al. Comparison of early postoperative morbidity after robot-assisted and open radical cystectomy: results of a prospective observational study. BJU Int. 2014; 113:458–467. PMID: 24053793.

62. Phillips EA, Uberoi V, Tuerk IA. Robot-assisted radical cystectomy in octogenarians. J Endourol. 2014; 28:219–223. PMID: 24074288.

63. Teishima J, Hieda K, Inoue S, Goto K, Ikeda K, Ohara S, et al. Comparison of initial experiences of robot-assisted radical cystectomy with those of laparoscopic for bladder cancer. Innovations (Phila). 2014; 9:322–326. PMID: 25062101.

64. Zeng S, Zhang Z, Yu X, Song R, Wei R, Zhao J, et al. Laparoscopic versus open radical cystectomy for elderly patients over 75-year-old: a single center comparative analysis. PLoS One. 2014; 9:e98950. PMID: 24901359.

65. Nix J, Smith A, Kurpad R, Nielsen ME, Wallen EM, Pruthi RS. Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: perioperative and pathologic results. Eur Urol. 2010; 57:196–201. PMID: 19853987.

66. Nguyen DP, Al Hussein Al Awamlh B, Charles Osterberg E, Chrystal J, Flynn T, Lee DJ, et al. Postoperative complications and short-term oncological outcomes of patients aged ≥80 years undergoing robot-assisted radical cystectomy. World J Urol. 2015; 33:1315–1321. PMID: 25410374.
67. Bochner BH, Sjoberg DD, Laudone VP;. A randomized trial of robot-assisted laparoscopic radical cystectomy. N Engl J Med. 2014; 371:389–390. PMID: 25054732.

68. Bochner BH, Dalbagni G, Sjoberg DD, Silberstein J, Keren Paz GE, Donat SM, et al. Comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: a randomized clinical trial. Eur Urol. 2015; 67:1042–1050. PMID: 25496767.

69. Smith ND, Castle EP, Gonzalgo ML, Svatek RS, Weizer AZ, Montgomery JS, et al. The RAZOR (randomized open vs robotic cystectomy) trial: study design and trial update. BJU Int. 2015; 115:198–205. PMID: 25626182.

70. Nayan M, Bhindi B, Yu JL, Mamdani M, Fleshner NE, Hermanns T, et al. The initiation of a multidisciplinary bladder cancer clinic and the uptake of neoadjuvant chemotherapy: a time-series analysis. Can Urol Assoc J. 2016; 10:25–30. PMID: 26977202.

71. Mathieu R, Lucca I, Klatte T, Babjuk M, Shariat SF. Trimodal therapy for invasive bladder cancer: is it really equal to radical cystectomy? Curr Opin Urol. 2015; 25:476–482. PMID: 26125510.
72. Rose TL, Milowsky MI. Management of muscle-invasive bladder cancer in the elderly. Curr Opin Urol. 2015; 25:459–467. PMID: 26075567.

73. Bassett JC, Chang SS. Treating octogenarians with muscle-invasive bladder cancer: preoperative opportunities for increasing the benefits of surgical intervention. Urol Oncol. 2014; 32:37.e13–37.e16. PMID: 23628313.

74. Hong NJ, Wright FC, Gagliardi AR, Paszat LF. Examining the potential relationship between multidisciplinary cancer care and patient survival: an international literature review. J Surg Oncol. 2010; 102:125–134. PMID: 20648582.

75. Galsky MD, Krege S, Lin CC, Hahn N, Ecke TH, Moshier E, et al. Cisplatin-based combination chemotherapy in septuagenarians with metastatic urothelial cancer. Urol Oncol. 2014; 32:30.e15–30.e21. PMID: 23428534.

76. Clayman RH, Shipley WU, Galland-Girodet S, Niemierko A, Gray PJ, Paly J, et al. Outcomes of selective bladder preservation in the elderly treated with conservative surgery and chemoradiation. Int J Radiat Oncol Biol Phys. 2013; 87(2 Suppl):S83.

77. Kouloulias V, Tolia M, Kolliarakis N, Siatelis A, Kelekis N. Evaluation of acute toxicity and symptoms palliation in a hypofractionated weekly schedule of external radiotherapy for elderly patients with muscular invasive bladder cancer. Int Braz J Urol. 2013; 39:77–82. PMID: 23489500.

78. Joudi FN, Smith BJ, O'Donnell MA, Konety BR. The impact of age on the response of patients with superficial bladder cancer to intravesical immunotherapy. J Urol. 2006; 175:1634–1639. PMID: 16600718.

79. Herr HW. Age and outcome of superficial bladder cancer treated with bacille Calmette-Guerin therapy. Urology. 2007; 70:65–68. PMID: 17656210.
80. Margel D, Alkhateeb SS, Finelli A, Fleshner N. Diminished efficacy of Bacille Calmette-Guérin among elderly patients with nonmuscle invasive bladder cancer. Urology. 2011; 78:848–854. PMID: 21840578.

81. Nazmy M, Yuh B, Kawachi M, Lau CS, Linehan J, Ruel NH, et al. Early and late complications of robot-assisted radical cystectomy: a standardized analysis by urinary diversion type. J Urol. 2014; 191:681–687. PMID: 24099746.

Fig. 1
Incidence of adverse events within 90 days by organ system. Adapted from Nazmy et al. J Urol 2014;191:681-7, with permission of Elsevier [81].
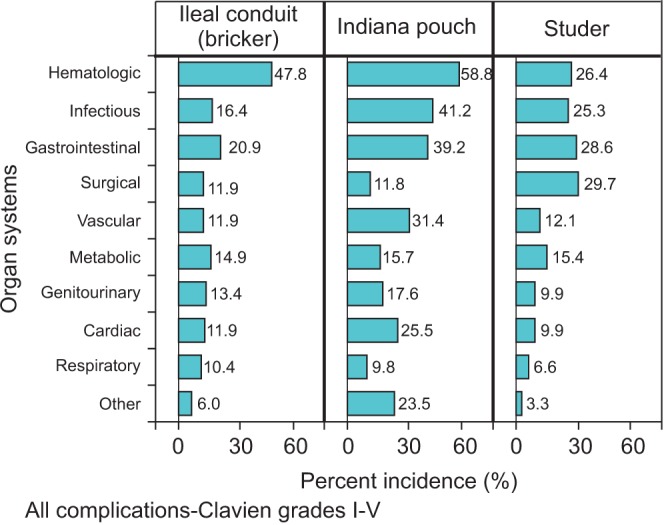
Table 1
Suggested treatment options for patients with muscle invasive bladder cancer
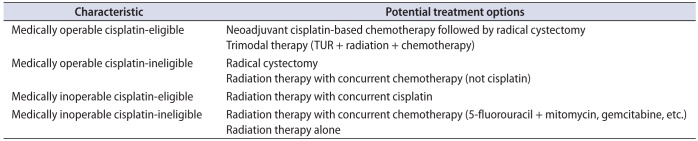
TUR, transurethral resection.
Adapted from Galsky. J Geriatr Oncol 2015;6:1-7, with permission of Elsevier [54].




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download