Abstract
Nerve-sparing techniques in robot-assisted radical prostatectomy (RARP) have advanced with the developments defining the prostate anatomy and robotic surgery in recent years. In this review we discussed the surgical anatomy, current nerve-sparing techniques and results of these operations. It is important to define the right and key anatomic landmarks for nerve-sparing in RARP which can demonstrate individual variations. The patients' risk assessment before the operation and intraoperative anatomic variations may affect the nerve-sparing technique, nerve-sparing degree and the approach. There is lack of randomized control trials for different nerve-sparing techniques and approaches in RARP, therefore accurate preoperative and intraoperative assessment of the patient is crucial. Current data shows that, performing the maximum possible nerve-sparing using athermal techniques have better functional outcomes.
According to the data obtained from the Surveillance, Epidemiology and End Results (SEER) registry, younger and healthier men are being diagnosed with localized prostate cancer every year with an annual percentage increase of 9.5% [1]. In younger patients, the functional outcomes (potency and continence) after radical prostatectomy are essential as well as the cancer control [23456789]. The surgical anatomy of the nerve-sparing radical prostatectomy was first described by Walsh and Donker in 1982, who also documented the concept of neurovascular bundle (NVB) and its relation with the prostate [10]. In this review, we aimed to discuss the current NVB anatomy; nerve-sparing techniques and functional results in robot-assisted radical prostatectomy (RARP).
Similar to all organs in the pelvis, the prostate is also covered by fascia (endopelvic fascia) which has 2 layers; parietal and visceral [11]. The parietal component (also named as the levator ani fascia) covers the levator ani muscles and the visceral component covers the pelvic organs including the prostate, the bladder and the rectum. The visceral part of the endopelvic fascia is fused with the anterior fibromuscular stroma of the prostate at the upper ventral wall of the prostate [1213] (Fig. 1A).
Some authors consider only the parietal component as the endopelvic fascia, whereas some others name the endopelvic fascia for the entire parietal and visceral components [141516]. The parietal and visceral components of the endopelvic fascia are fused at the lateral aspect of the prostate and the bladder. This area is observed as a whitish line which is named as arcus tendinous fascia pelvis, and extend from the puboprostatic and pubovesical ligaments of the ischial spine (Fig. 1B, C). Some authors suggested that without opening the endopelvic fascia during RARP in intrafascial dissection, the functional outcomes such as continence and potency might be improved [1516].
The periprostatic fascia contains all fascias on the prostate which are external to the prostatic capsule. Today there is still controversy in nomenclature. Thus, this fascia is named as lateral pelvic fascia, parapelvic fascia and prostatic fascia [17]. The periprostatic fascia is divided into 3 basic parts according to its location (Fig. 2).
This part of the periprostatic fascia covers the anterior surface of the prostate from 10 o'clock to 2 o'clock position in the clockwise direction. This part also covers the detrusor apron, the dorsal vascular complex and is fused with the anterior fibromuscular stroma of the prostatic stroma (Fig. 2A, B).
During RARP, if the endopelvic fascia is incised laterally to the arcus tendinous fascia, the pelvis and the levator ani muscle are deflected laterally (Fig. 1). Levator ani fascia is the outermost fascial layer of the prostate laterally. In most cases, there is an inner fascia called prostatic fascia which is usually multilayered [1819]. Both these layers; the levator ani fascia and the prostatic fascia constitute the periprostatic fascia. The layers of this fascia extend from the anterior surface of the prostate to the posterior side of prostate. Hence the pararectal fascia is formed by the lateral periprostatic fascia and the levator ani fascia posteriorly near the lateral side of the NVB. The pararectal fascia separates the levator ani muscle from the rectum [112021]. The inner prostatic fascia passes medially to the NVB and is lateral to the prostatic capsule.
The anatomy of the prostatic capsule and the inner part of the prostatic fascia may differ individually. Kiyoshima et al. [22] reported that the levator ani fascia which is called lateral pelvic fascia is not adhered to the prostatic capsule in 52% of all cases. The space between the prostatic capsule and the inner part of the prostatic fascia consists of loose connective tissue and adipose tissue named as the areolar tissue. In such cases, NVB could not be identified as a separate structure but was spread to the lateral surface of the prostate as a spray like formation (Fig. 3A) [22].
The Denonvilliers' fascia covers the posterior surface of the prostate and the seminal vesicles. Some authors consider this fascia as the fusion of the embryonic peritoneum of the rectovesical cul-de-sac, while others consider that the Denonvilliers' fascia is the anterior part of the remnant of 2 fused peritoneal layers [172324]. This fusion of layers enables a prerectal cleavage; an anatomic plane between the rectal fascia and the Denonvilliers' fascia. The cephalad origin of this fascia is in the anterior part of the peritoneal cul-de-sac which is named as the recto vesical pouch. The Denonvilliers' fascia extends to the apex of the prostate and ends at the prostatourethral junction as a plate in the central perineal surface [24]. In fact, the posterior layer of the Denonvilliers' fascia is the thin fascia serosa of the rectum. The Denonvilliers' fascia is often fused with prostate at the centre posteriorly. It has no significant fusion with the prostate capsule at the posterolateral wall so this space is filled with the areolar tissue and during the nerve-sparing, the posterior dissection can easily be performed (Figs. 3B, 4) [1724].
Some authors reported that the NVB is located between the prostatic capsule, the Denonvilliers' fascia and the lateral prostatic fascia [2325]. There are no nerve fibers which are lateral to the levator ani fascia and dorsal to the Denonvilliers' fascia [2126]. It is also reported that only two-thirds of all nerves are on the lateral surface of the posterolateral location and the remaining are on the anterolateral surface (Fig. 5) [2127].
Kourambas et al. [20] reported that, in the axial plane, the Denonvilliers' fascia is exactly an H-shaped fascial structure (Fig. 3B). The upper limbs the ‘H’ are formed bilaterally by the periprostatic fascia and the lower limbs of the ‘H’ by the pararectal fascia; and the horizontal bar of the ‘H’ is identified by the Denonvilliers' fascia. Moreover they also reported that the Denonvilliers' fascia was not clearly seen at the lateral edges where all three fascias were interconnecting. They demonstrated the lateral divisions of the Denonvilliers' fascia as several layers where neural fibres were found and the nerve fibres are located at both ventral and dorsal surfaces of the Denonvilliers' fascia. The periprostatic fascia layers fuse with the anterior layer of the Denonvillier' s fascia laterally to the prostate, and form a triangular space surrounding NVB; the medial wall is composed by periprostatic fascia, the lateral wall by lateral pelvic fascia (levator fascia) and the posterior wall by anterior layer of Denonvilliers' fascia. This triangular space is wide near the base and narrow at the apex of the prostate (Fig. 3B).
The prostate does not have a real capsule but it is covered by a capsule-like structure which is formed by a compact layer of fibromuscular smooth muscle. Multiple vessels and nerves penetrate into the capsule from the lateral wall of the prostate [1322]. The capsule of the prostate usually cannot be seen from the anterior of prostate base which has a detrusor apron at this level [13]. At the base of the prostate, the capsule is fixed to the muscular fibres of the bladder detrusor (Fig. 2) [28].
Walsh and Donker [10] demonstrated that the pelvic plexus is formed by a parasympathetic cul-de-sac (S2–S4 anterior sacral roots) and by the sympathetic fibres from the hypogastric nerve. Pelvic plexus is located at the anterolateral wall of the rectum retroperitoneally, near the tips of the seminal vesicles and the posterolateral wall of the prostate [1029]. Costello et al. [21] demonstrated that the nerve fibres supplying the urinary sphincter and the corpora cavernosa, are primarily located posteriorly at the pelvic plexus.
Tewari et al. [30], after the completion of their cadaver study, described tri-zonal neural architecture model. Zone 1 is located at the proximal zone containing the proximal neurovascular plate which covers a significant portion of the proximal prostate on the lateral wall and is connected to the bladder neck and the seminal vesicles. Zone 1 is 5–10 mm lateral to the seminal vesicles and is at risk mostly of thermal injury or crushing by clips and bull dog clamps. Zone 2 is located at the posterolateral groove of the prostate which comprises the predominant NVB. This part of the bundle is well developed in 50% of the patients and is widely spread in the periprostatic region in the other 50%. Zone 2 is closely related to prostate pedicles and fascia, thus identification is difficult and could be damaged because of the periprostatic inflammation and the extracapsular extension. Zone 3 is the distal zone, which contains apical nerves and the accessory pathways. It was reported in the study that the accessory nerves may be found around the prostate, between the lateral pelvic fascia, the prostatic fascia and the Denonvilliers' fascia. In apical dissection, retroapical nerves can be injured during urethral transection and anastomosis.
During the cavernosal nerves' course lateral to the prostate, the studies demonstrated a spray like distribution of the nerves on the lateral and the anterolateral surface of the prostate up to 2 o'clock and 10 o'clock positions [2231]. Ganzer et al. [31], using computerized planimetry, reported that the largest percentage of periprostatic nerves is present in the posterolateral position. The cavernosal nerve distribution was variable, up to 19% of the overall nerves were in the anterolateral position [31]. Alsaid et al. [32] confirmed this finding in their study proving with immunohistochemical confirmation of 3-dimensional reconstruction. They found that at the mid part of the NVB, the nerves became more spread, with less than two-thirds of the periprostatic nerve fibres remaining in the posterolateral regions and one-third in the anterior and anterolateral regions. At the apex, 60% of the nerves were located posterolaterally and 40% were located anterolaterally [32]. In another study, Clarebrough et al. [33] showed that the overall proportion of the nerve surface increased at the anterolateral side of the prostate, from 6% at the base to 7.6% at the mid part and to 11.2% at the apex; especially at the apex nerve fibres were more predominant along the anterolateral side of the prostate. Walz et al. [34] evaluated these studies as having conflicting results which can be explained by the “interindividual variability” of the anatomy and also by the difference in methodology of the nerve surface and the number of nerve fibers.
There are numerous different nerve-sparing surgical techniques and approaches in RARP and these techniques are mainly related to the surgical anatomy of periprostatic fascias. The degree of fusion between the lateral pelvic fascia and the prostate capsule affects the site and localization of NVB. The intrafascial plane is the plane between the prostate capsule and the prostatic fascia. The interfascial plane is the plane between the prostatic fascia and the lateral pelvic fascia (the levator fascia) (Fig. 6). Before starting intrafascial or interfascial dissection plane endopelvic fascia must be incised on the arcus tendineous fascia pelvis as shown in Fig. 1B. The extrafascial plane is defined from the external part of the NVB and is a non–nerve-sparing technique. Therefore the preservation of the NVB can be achieved by either interfascial or intrafascial dissection [3536373839]. The Pasadena consensus panel suggested alternate terminology of dissection planes as full, partial and minimal nerve-sparing for the intrafascial, the interfascial and the subextrafascial dissections respectively (Fig. 7A) [40].
Nerve-sparing technique in RARP is crucial for postoperative potency and continence improvement. However there is an increased risk of positive surgical margins following the nerve-sparing techniques especially during the surgical learning curve [34]. Several studies reported significant positive association between the nerve-sparing techniques with the positive surgical margins, conversely some other studies reported no significant association [35414243]. Nerve-sparing techniques especially carry out a dissection plane of posterolateral dissection very close to the prostate and the tumor at the base, therefore the surgeon has to be careful for positive surgical margins (Fig. 8) [44]. Posterior dissection of Denonvilliers' fascia up to prostate apex is mandatory for a high quality nerve-sparing except any contraindications (Fig. 4).
Because of the interindividual and intraindividual variations, the surgeons usually, do not do the same surgical dissection plane in every patient. The multilayered character of the periprostatic fascia allows choices in the dissection between the nerves and the prostate capsule. For the cases with a low risk of extraprostatic extension (EPE), the surgeons may perform a closer dissection while for the cases with a higher risk of EPE, the surgeons may prefer a wider dissection plane. This approach was introduced as incremental nerve-sparing [4045]. In most cases, EPE is only found a few millimetres away from the prostate that could allow the nerve-sparing procedure in well-selected patients with focal EPE. The previous study by Inoue et al. [46] evaluated the distance between the cancer and the NVB at 5 o' clock and 7 o' clock positions without the nerve-sparing. In patients without EPE, they reported a mean distance of 3.3 mm, 3.4 mm, and 3.7 mm at the apex, the midgland and the base respectively. In patients with EPE, the distances between the cancer and the NVB were 2.0 mm, 1.9 mm, and 1.8 mm at the apex, the midgland, and the base, respectively. The major factor that affects the distance between the tumor and NVB was the “tumor size” in the apex and the base of the prostate [46]. This observation supports the applicability of the nerve-sparing procedures despite the presence of EPE in well selected patients [34]. Multiparametric prostate magnetic resonance imaging may be a useful tool for determining the EPE and the NVBs before the surgery according to our experience for choosing the right dissection plane as an incremental nerve-sparing approach [45].
The intrafascial dissection of the NVB is a plane that follows on the capsule, remaining internally in the prostatic fascia at the anterolateral and posterolateral aspect of the prostate and the anterior to the Denonvilliers' fascia (Figs. 5, 6). The intrafascial dissection allows the total preservation of the NVB. However, the intrafascial dissection carries out the greatest risk of iatrogenic capsular damage.
In the antegrade approach of the intrafascial dissection starting from the 6 o'clock position, the surgeon may perform an easier plane because the Denonvilliers' fascia is thicker and is as a single layer at this level (Fig. 4). During a high lateral approach, this plane can be more difficult to dissect due to the multilayered position of the fascias mainly at the posterolateral side of the prostate [17].
The interfascial dissection of the NVB is a plane between the leaves of the prostatic fascia and includes incremental nerve-sparing which is a partial resection of the NVB. The interfascial dissection plane is between the lateral parts of the prostatic fascia at the anterolateral and posterolateral sides of the prostate and the medial side of NVB (Fig. 6). The lateral prostatic fascia stays on the prostate specimen rather than on the NVB [17].
The approach to the nerve-sparing technique in RARP can be antegrade; from prostate base to the apex or retrograde; from the apex to the base of the prostate. Unfortunately there is a lack of sufficient data to define the better surgical approach for the nerve-sparing; the antegrade or the retrograde?
After the upward traction of vas and seminal vesicles, the prostatic pedicle is observed and controlled athermally at the base of the prostate. Then the prostate is pulled to the opposite side and the lateral pelvic fascia is exposed. The triangular space between the lateral pelvic fascia, the Denonvilliers' fascia and the prostate is observed and the NVB is defined. The lateral pelvic fascia is exposed and the interfascial or the intrafascial dissection is performed (Fig. 9A).
After the dissection of the seminal vesicles and the posterior plane is developed up distally, the prostate is rotated to the left or to the right opposite side. The lateral pelvic fascia is opened sharply to expose the NVB at mid prostate level. Then the dissection is performed to the posterior plane until releasing the NVB from the prostate pedicle. After controlling the prostatic pedicles with Hem-o-lok clips at the base, the dissection is carried on antegradely to the apex to release full NVB from the prostate (Fig. 10). Complete release of the NVB at the apex is important for avoiding any injury in apical dissection (Fig. 9B).
The retrograde approach has advantages for earlier identification and the release of the NVB from the prostate before controlling the pedicle, it is commented as this approach lead to avoid a misdisplaced clip on the pedicle and reduced neuropraxia [47]. Only the nonrandomized comparative study from Ko et al. [47] reported that patients with the retrograde approach for nerve-sparing in RARP had significantly earlier potency recovery than the antegrade group.
Kaul et al. [48] from Vattikuti Urology Institute reported the technique known as the Veil of Aphrodite. In this technique, they dissected and made a space between the prostate capsule and the fascia starting from the base of the seminal vesicles by using curved shears of the Harmonic ACE. Then, they performed interfascial dissection between 1 and 5 o'clock for the right and 6 and 11 o'clock for the left athermally. With their antegrade dissection, at the end, the curtains of the periprostatic tissue hung from pubourethral ligaments, and it is mentioned as the veil of Aphrodite [18]. Menon et al. [43] reported that they modified the original veil technique by extending the interfascial dissection anteriorly which (super veil technique) was recommended for only in low-risk patients.
Another study from Chien et al. [49] reported the technique of modified clipless antegrade nerve-sparing RARP without monopolar cautery. After the bladder neck division, they dissected the posterior plane of the prostate distally to the apex in the midline route. After controlling the vascular pedicles and NVB from the medial to the lateral direction, the plane was performed completely without the use of clips and the monopolar cautery, but when necessary only the bipolar cautery was used.
Badani et al. [50] used the laparoscopic doppler ultrasound to identify the NVB during RARP in a pilot study and concluded that it was a safe, easy and effective method to get a greater nerve-sparing.
The nerve-sparing may be performed using thermal instruments such as monopolar, bipolar, harmonic cauteries by keeping in mind that thermal damage could be observed. Ahlering et al. [51] reported that the potency rates were significantly higher in non-cautery bilateral nerve-sparing group than the cautery used group (92% vs. 67.9% during 24 months). They also did not use the clips for the haemorrhage of NVB, and applied alternative hemostatic agents. On a similar approach by the same team, local hypothermia was applied during RARP with endorectal cooling balloon system and they reported the achievement of a significantly early continence with this approach [52].
Patel et al. [53] reported the first study about preoperatively potent 58 cases who had bilateral nerve-sparing RARP by the application of dehydrated human amniotic membrane as the wrap on NVB. In short term results, the potency and the continence rates were found significantly higher than the other group. And they recommend using dehydrated human amniotic membrane to improve the early return of the continence and the potency.
Some authors described the nerve-sparing techniques as anatomic grading according to NVB vascular landmarks in selected patients. Tewari et al. [45] suggested a grading system which was based on 4 grades of dissection. They used the veins on the lateral aspect of the prostate as landmarks for description of the dissection planes such as grade 1 is the maximum nerve-sparing and grade 4 is the non–nerve-sparing (Figs. 7B, 9B; Table 1). According to this system, grade 1 is the dissection plane between the periprostatic veins and the prostate capsule. The dissection which is carried out just on the veins, is grade 2 dissection. When leaving more adipose and neural tissue on the veins and the prostate, it is grade 3 dissection plane while extrafascial dissection is grade 4. They reported that the patients with greater degrees of nerve-sparing had higher rates of intercourse without an increase in surgical margin positivity [45]. By using this grading system, early return of continence with grade 1 dissection was 72% while this was 44% with grade 4 [54].
The other grading system which was reported by Schatloff et al. [55] in 2012, in this grading system grade 5 was optimal nerve-sparing and grade 1 was non–nerve-sparing (Figs. 7C, 9B; Table 1). They used the NVB artery as the landmark that was named “landmark artery” running on the lateral wall of the prostate (Fig. 11). The landmark artery was often found between the mid prostate and the base, as a tortuous artery. The landmark arteries were identified at 73% of any operated sides of all cases [56]. The dissection plane is performed between the landmark artery and the prostate at the level of mid prostate using Maryland dissector and scissors. In the right plane the dissection is simple as a natural surgical plane. The plane is continued retrogradely up to the posterior plane and the base of the prostate. After controlling the prostatic pedicles at the base, dissection is carried on the antegrade of the apex. In this system maximum nerve-sparing was named as grade 5 dissection and the dissection was performed between the landmark artery and the capsule outside of the prostatic fascia without sharp dissection. Grade 4 dissection was performed using sharp dissection in a plane between the artery and the prostate capsule. This plane was confirmed by the presence of a strip of adipose tissue over the prostate and the absence of the arterial vessels. For the grade 3 dissection, the plane was made laterally to the landmark artery, hence the artery was clipped at the level of the prostate pedicle. The dissection is identified intraoperatively by the presence of a strip of adipose tissue over the prostate with the artery on top. In the grade 2 dissection, nerve-sparing was completed several millimetres laterally to the artery following the prostate curve. The grade 2 dissection was identified intraoperatively by the presence of a thick fat strip over the prostate with arteries embedded. Grade 1 dissection was an extrafascial plane. They reported that with increasing degrees of the nerve-sparing, the amount of nerve tissue on the prostate decreased (Fig. 7C).
There is limited data for this issue; only one study from Finley et al. [57] reported no significant difference in unilateral and bilateral nerve-sparing in RARP. But this study mainly comparing the cautery and cautery-free patients, and unilateral patient population in the study was lower than the bilateral group. Making a clear decision with this study's conclusion is not sufficient at the moment. The study which comparing the unilateral and bilateral intrafascial nerve-sparing in a laparoscopic radical prostatectomy (LRP), reported that patients with bilateral nerve-sparing had significantly high rates of ability to engage sexual intercourse than unilateral group [58]. While maximum nerve-sparing is recommended for functional outcomes after RARP, studies comparing unilateral and bilateral nerve-sparing in RARP is needed in future.
In comparative studies between the RARP and the open radical retropubic prostatectomy (RRP) series, most of them had significant advantages of potency recovery in RARP while some other studies did not find any differences in either functional or oncological outcomes [596061]. On the comparative series for RARP and the LRP, RARP was reported to have significant high potency rates than the LRP or comparable results (Table 2) [578].
The potency rates of larger series in RARP are listed in Table 3. The potency rates changes from 54% to 97.4% in different studies [21835364142476263]. These wide ranges of potency rates could be because of the inclusion criteria, the evaluation methods, different nerve-sparing techniques and approaches, the surgeons' experience and the follow-up periods. Moreover, the definition of potency is not standardised in most of the studies. But it is obvious that the potency in nerve-sparing RARP series is determined to be higher not only in the short term results but also in the long term results for the RRP and the LRP.
Nerve-sparing in RARP is the one of the main goals of the radical prostatectomy operations in which the patient risk is stratified preoperatively. However, during the nerve-sparing, it is strongly recommended to use the athermal dissection and the athermal control of the prostatic pedicle, not to have any or to have a minimal traction to the NVB and bilateral nerve-sparing as much as possible. Recent data suggest that the RARP has better functional outcomes than the RRP and the LRP without compromising the oncological results. Current surgical techniques for nerve-sparing RARP are essentially affected by the patients' preoperative potency, the preoperative risk assessments of biopsy results, the tumor extent and mostly the surgical anatomy of the patient. Nerve-sparing RARP has involved different surgical techniques such as intrafascial or interfascial techniques and different surgical approaches such as the antegrade or the retrograde approaches; meanwhile there is a lack of randomized controlled trials to define which technique is superior to another. The patients' individual anatomic factors may affect these techniques and approaches, therefore, the surgeon's experience is important to decide the right surgical technique intraoperatively which is called incremental nerve-sparing. According to the patients' anatomy, choosing the right surgical dissection planes are crucial for the convenient nerve-sparing RARP.
Figures and Tables
Fig. 1
(A) Visceral component (yellow arrow) and parietal component (blue arrow) of the endopelvic fascia, arcus tendineus fascia pelvis (red dashed line). (B) Dissection of interfascial and intrafascial (nerve-sparing) plane. (C) Dissection of extrafascial plane, levator ani muscle (red arrow).
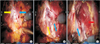
Fig. 2
(A) Axial section of prostatic and periprostatic fascia at midprostate. (B) Midline sagittal section of prostate, bladder and striated sphincter. AFMS, anterior fibromuscular stroma; B, bladder; CS, colliculus seminalis (verumontanum); DA, detrusor apron; DVC, dorsal vascular complex; FTAP, fascial tendineus arch of pelvis; LAF, levator ani fascia; MDR, medial dorsal raphe; NVB, neurovascular bundle; PC, pseudocapsule of prostate; PPF, periprostatic fascia; PPF/SVF, posterior prostatic fascia/seminal vesical fascia; PRS, perirectal space; PS, pubic symphysis; PZ, peripheral zone; R, rectum; RU, rectourethralis muscle; SMS, smooth muscle sphincter; SS, striated sphincter; SV, seminal vesicle; TZ, transition zone; U, urethra; VEF, visceral endopelvic fascia; VPM, vesicoprostatic muscle; VS, vesical sphincter. Adapted from Walz et al. Eur Urol 2016;70:301-11, with permission of Elsevier B.V. [34].
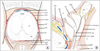
Fig. 3
(A) Coronal section of prostate, sphincteric urethra, periprostatic fascias and associated musculature. (B) Axial section through base of seminal vesicles to neurovascular bundle (NVB). B, bladder; M, midprostate; A, apex; U, urethra; CS, colliculus seminalis; CZ, central zone; LAF, levator ani fascia; PPF, periprostatic fascia; PC, pseudocapsule of prostate; ED, ejaculatory duct; LA, levator ani muscle; PF, prostatic fascia; PP, pelvic plexus; PPF/SVF, posterior prostatic fascia/ seminal vesicle fascia (Denonvilliers fascia); PZ, peripheral zone; R, rectum; SMS, smooth muscle sphincter; SS, striated sphincter; SV, seminal vesicle; VD, vas deferens; VPM, vesicoprostatic muscle. Adapted from Walz et al. Eur Urol 2010;57:179-92, with permission of Elsevier B.V. [17].
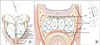
Fig. 4
Posterior prostatic dissection plane. Yellow arrow means vas deferens; blue arrows mean Denonvillier fascia; asterisk means seminal vesicles.
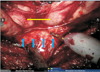
Fig. 5
Right intrafascial dissection; prostate and right neurovascular bundle (NVB). Yellow arrows mean dissection plane; blue arrow means NVB; asterisk means prostate capsule.
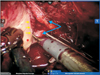
Fig. 6
Schematic drawing of 2 different surgical techniques for nerve-sparing prostatectomy. (A) Interfascial nerve-sparing prostatectomy. (B) Intrafascial nerve-sparing prostatectomy. EF, endopelvic fascia; PF, periprostatic fascia; PC, prostatic capsula; PP, prostatic pedicle; NBV, neurovascular bundle. Adapted from Stolzenburg et al. Eur Urol 2007:51:629-39, with permission of Elsevier B.V. [39].
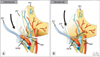
Fig. 7
(A) Three dissection planes according to the Pasadena consensus [45], (B) Four dissection planes according to Tewari et al. [45], 1, dissection below veins, 2, dissection on the veins, 3, dissection distant from the veins, 4, extrafascial dissection, (C) Five dissection planes according to Schatloff et al. [55]: 1, extrafascial dissection; 2, sharp dissection distant from arteries; 3, sharp dissection on arteries; 4, sharp dissection on the level of arteries; 5, blunt dissection below arteries. Adapted from Walz et al. Eur Urol 2016;70:301-11, with permission of Elsevier B.V. [34].
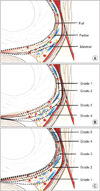
Fig. 8
View of prostate specimen, intrafascial dissection on right side, interfascial dissection on left side. Asterisk means prostate capsule; yellow line means posterior midline.
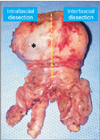
Fig. 9
(A) Antegrade neurovascular bundle (NVB) dissection on right side, (B) retrograde NVB dissection on left side. Yellow star means prostatic pedicle; blue arrow means prostate capsule; red dashed line and arrow mean NVB.
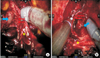
Fig. 11
Right landmark artery on right intrafascial dissection. Yellow arrows mean right landmark artery.
Table 1
Different dissection planes of nerve-sparing RARP; and their influence on safety margin and nerve-sparing quality

| Author | Dissection plane | |||||||
|---|---|---|---|---|---|---|---|---|
|
Safety margin to avoid positive surgical margin Low<---------------------------------------------------------------------------------->High |
||||||||
|
Nerve-sparing quality Good<-------------------------------------------------------------------------------->Poor |
||||||||
| Walz et al. [34] | Intrafascial | Interfascial | Extrafascial | |||||
| Montorsi et al. [40] | Full nerve sparing | Partial nerve sparing | Minimal nerve sparing | Not applicable | ||||
| Tewari et al. [45] | Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||||
| Schatloff et al. [55] | Grade 5 | Grade 4 | Grade 3 | Grade 2 | Grade 1 | |||
RARP, robot-assisted radical prostatectomy. Adapted from Walz J et al. Eur Urol 2016;70:301-11, with permission of Elsevier B.V. [34].
Table 2
Potency outcomes in comparative series of RRP, LRP, and RARP

| Study | Comparison | No. | Mean age (y) | NS technique | Potency rate (overall) |
|---|---|---|---|---|---|
| Ficarra et al. [59] | RARP vs. RRP | 103 vs. 105 | 61 vs. 65 | Antegrade | 81% vs. 49% |
| Rocco et al. [60] | RARP vs. RRP | 120 vs. 240 | 63 vs. 63 | Retrograde | 61% vs. 41% |
| Krambeck et al. [61] | RARP vs. RRP | 294 vs. 588 | 61 vs. 61 | Antegrade | 70% vs. 62% |
| Willis et al. [5] | RARP vs. LRP | 121 vs. 161 | 58.1 vs. 58 | Antegrade | 87.5% vs. 66.7% |
| Porpiglia et al. [8] | RARP vs. LRP | 60 vs. 60 | 63.9 vs. 64.7 | Antegrade | 80% vs. 54.2% |
| Berge et al. [7] | RARP vs. LRP | 210 vs. 210 | 61.7 vs. 61.7 | Antegrade | 61.3% vs. 57.3% |
RARP, robot-assisted radical prostatectomy; RRP, open radical retropubic prostatectomy; LRP, laparoscopic radical prostatectomy.
Adapted from Kumar et al. J Robot Surg 2016;10:187-200, with permission of Springer [44].
Table 3
Potency outcomes in noncomparative series of RARP

| Study | No. | Nerve sparing approach | Mean age (y) | Potency rate (overall) |
|---|---|---|---|---|
| Menon et al. [18] | 721 | Antegrade | 60.2 | 70% |
| Shikanov et al. [36] | 703 | Antegrade | 58.5 | 64% |
| Potdevin et al. [35] | 147 | Retrograde | 58.7 | 90% |
| Patel et al. [42] | 404 | Retrograde | 58 | 97.4% |
| Patel et al. [62] | 332 | Retrograde | 58.5 | 89.8% |
| Kowalczyk et al. [63] | 342 | Antegrade | 59.6 | 50% |
| Alemozaffar et al. [41] | 400 | Retrograde | 59.8 | 59.3% |
| Ficarra et al. [3] | 183 | Antegrade | 62.3 | 82% |
| Ko et al. [47] | 172 vs. 172 | Antegrade vs. retrograde | 57.9 vs. 57.2 | 85.3% vs. 92.9% |
RARP, robot-assisted radical prostatectomy.
Adapted from Kumar et al. J Robot Surg 2016;10:187-200, with permission of Springer [44].
References
1. Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, et al. Annual Report to the Nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014; 120:1290–1314.
2. Ficarra V, Borghesi M, Suardi N, De Naeyer G, Novara G, Schatteman P, et al. Long-term evaluation of survival, continence and potency (SCP) outcomes after robot-assisted radical prostatectomy (RARP). BJU Int. 2013; 112:338–345.
3. Ficarra V, Novara G, Artibani W, Cestari A, Galfano A, Graefen M, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2009; 55:1037–1063.
4. Coelho RF, Rocco B, Patel MB, Orvieto MA, Chauhan S, Ficarra V, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a critical review of outcomes reported by high-volume centers. J Endourol. 2010; 24:2003–2015.
5. Willis DL, Gonzalgo ML, Brotzman M, Feng Z, Trock B, Su LM. Comparison of outcomes between pure laparoscopic vs robot-assisted laparoscopic radical prostatectomy: a study of comparative effectiveness based upon validated quality of life outcomes. BJU Int. 2012; 109:898–905.
6. Asimakopoulos AD, Miano R, Di Lorenzo N, Spera E, Vespasiani G, Mugnier C. Laparoscopic versus robot-assisted bilateral nerve-sparing radical prostatectomy: comparison of pentafecta rates for a single surgeon. Surg Endosc. 2013; 27:4297–4304.
7. Berge V, Berg RE, Hoff JR, Wessel N, Diep LM, Karlsen SJ, et al. A prospective study of transition from laparoscopic to robot-assisted radical prostatectomy: quality of life outcomes after 36-month follow-up. Urology. 2013; 81:781–786.
8. Porpiglia F, Morra I, Lucci Chiarissi M, Manfredi M, Mele F, Grande S, et al. Randomised controlled trial comparing laparoscopic and robot-assisted radical prostatectomy. Eur Urol. 2013; 63:606–614.
9. Asimakopoulos AD, Pereira Fraga CT, Annino F, Pasqualetti P, Calado AA, Mugnier C. Randomized comparison between laparoscopic and robot-assisted nerve-sparing radical prostatectomy. J Sex Med. 2011; 8:1503–1512.
10. Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982; 128:492–497.
11. Takenaka A, Hara R, Soga H, Murakami G, Fujisawa M. A novel technique for approaching the endopelvic fascia in retropubic radical prostatectomy, based on an anatomical study of fixed and fresh cadavers. BJU Int. 2005; 95:766–771.
12. Savera AT, Kaul S, Badani K, Stark AT, Shah NL, Menon M. Robotic radical prostatectomy with the "Veil of Aphrodite" technique: histologic evidence of enhanced nerve sparing. Eur Urol. 2006; 49:1065–1073.
13. Ayala AG, Ro JY, Babaian R, Troncoso P, Grignon DJ. The prostatic capsule: does it exist? Its importance in the staging and treatment of prostatic carcinoma. Am J Surg Pathol. 1989; 13:21–27.
14. Myers RP, Villers A. Anatomic considerations in radical prostatectomy. In : Kirby RS, Partin AW, Feneley M, Parsons JK, editors. Prostate cancer: principles and practice. London: Taylor & Francis;2006. p. 701–713.
15. Stolzenburg JU, Rabenalt R, Do M, Schwalenberg T, Winkler M, Dietel A, et al. Intrafascial nerve-sparing endoscopic extraperitoneal radical prostatectomy. Eur Urol. 2008; 53:931–940.
16. Tewari AK, Bigelow K, Rao S, Takenaka A, El-Tabi N, Te A, et al. Anatomic restoration technique of continence mechanism and preservation of puboprostatic collar: a novel modification to achieve early urinary continence in men undergoing robotic prostatectomy. Urology. 2007; 69:726–731.
17. Walz J, Burnett AL, Costello AJ, Eastham JA, Graefen M, Guillonneau B, et al. A critical analysis of the current knowledge of surgical anatomy related to optimization of cancer control and preservation of continence and erection in candidates for radical prostatectomy. Eur Urol. 2010; 57:179–192.
18. Menon M, Shrivastava A, Kaul S, Badani KK, Fumo M, Bhandari M, et al. Vattikuti Institute prostatectomy: contemporary technique and analysis of results. Eur Urol. 2007; 51:648–657.
19. Nielsen ME, Schaeffer EM, Marschke P, Walsh PC. High anterior release of the levator fascia improves sexual function following open radical retropubic prostatectomy. J Urol. 2008; 180:2557–2564.
20. Kourambas J, Angus DG, Hosking P, Chou ST. A histological study of Denonvilliers' fascia and its relationship to the neurovascular bundle. Br J Urol. 1998; 82:408–410.
21. Costello AJ, Brooks M, Cole OJ. Anatomical studies of the neurovascular bundle and cavernosal nerves. BJU Int. 2004; 94:1071–1076.
22. Kiyoshima K, Yokomizo A, Yoshida T, Tomita K, Yonemasu H, Nakamura M, et al. Anatomical features of periprostatic tissue and its surroundings: a histological analysis of 79 radical retropubic prostatectomy specimens. Jpn J Clin Oncol. 2004; 34:463–468.
23. Lindsey I, Guy RJ, Warren BF, Mortensen NJ. Anatomy of Denonvilliers' fascia and pelvic nerves, impotence, and implications for the colorectal surgeon. Br J Surg. 2000; 87:1288–1299.
24. Villers A, McNeal JE, Freiha FS, Boccon-Gibod L, Stamey TA. Invasion of Denonvilliers' fascia in radical prostatectomy specimens. J Urol. 1993; 149:793–798.
25. van Ophoven A, Roth S. The anatomy and embryological origins of the fascia of Denonvilliers: a medico-historical debate. J Urol. 1997; 157:3–9.
26. Lepor H, Gregerman M, Crosby R, Mostofi FK, Walsh PC. Precise localization of the autonomic nerves from the pelvic plexus to the corpora cavernosa: a detailed anatomical study of the adult male pelvis. J Urol. 1985; 133:207–212.
27. Lunacek A, Schwentner C, Fritsch H, Bartsch G, Strasser H. Anatomical radical retropubic prostatectomy: 'curtain dissection' of the neurovascular bundle. BJU Int. 2005; 95:1226–1231.
28. Epstein JI. Pathologic assessment of the surgical specimen. Urol Clin North Am. 2001; 28:567–594.
29. Quinlan DM, Epstein JI, Carter BS, Walsh PC. Sexual function following radical prostatectomy: influence of preservation of neurovascular bundles. J Urol. 1991; 145:998–1002.
30. Tewari A, Takenaka A, Mtui E, Horninger W, Peschel R, Bartsch G, et al. The proximal neurovascular plate and the tri-zonal neural architecture around the prostate gland: importance in the athermal robotic technique of nerve-sparing prostatectomy. BJU Int. 2006; 98:314–323.
31. Ganzer R, Blana A, Gaumann A, Stolzenburg JU, Rabenalt R, Bach T, et al. Topographical anatomy of periprostatic and capsular nerves: quantification and computerised planimetry. Eur Urol. 2008; 54:353–360.
32. Alsaid B, Bessede T, Diallo D, Moszkowicz D, Karam I, Benoit G, et al. Division of autonomic nerves within the neurovascular bundles distally into corpora cavernosa and corpus spongiosum components: immunohistochemical confirmation with three-dimensional reconstruction. Eur Urol. 2011; 59:902–909.
33. Clarebrough EE, Challacombe BJ, Briggs C, Namdarian B, Weston R, Murphy DG, et al. Cadaveric analysis of periprostatic nerve distribution: an anatomical basis for high anterior release during radical prostatectomy? J Urol. 2011; 185:1519–1525.
34. Walz J, Epstein JI, Ganzer R, Graefen M, Guazzoni G, Kaouk J, et al. A critical analysis of the current knowledge of surgical anatomy of the prostate related to optimisation of cancer control and preservation of continence and erection in candidates for radical prostatectomy: An Update. Eur Urol. 2016; 70:301–311.
35. Potdevin L, Ercolani M, Jeong J, Kim IY. Functional and oncologic outcomes comparing interfascial and intrafascial nerve sparing in robot-assisted laparoscopic radical prostatectomies. J Endourol. 2009; 23:1479–1484.
36. Shikanov S, Woo J, Al-Ahmadie H, Katz MH, Zagaja GP, Shalhav AL, et al. Extrafascial versus interfascial nerve-sparing technique for robotic-assisted laparoscopic prostatectomy: comparison of functional outcomes and positive surgical margins characteristics. Urology. 2009; 74:611–616.
37. Martinez-Salamanca JI, Ramanathan R, Rao S, Mandhani A, Leung R, Horninger W, et al. Second Prize: Pelvic neuroanatomy and innovative approaches to minimize nerve damage and maximize cancer control in patients undergoing robot-assisted radical prostatectomy. J Endourol. 2008; 22:1137–1146.
38. Carter S, Le JD, Hu JC. Anatomic and technical considerations for optimizing recovery of sexual function during robotic-assisted radical prostatectomy. Curr Opin Urol. 2013; 23:88–94.
39. Stolzenburg JU, Schwalenberg T, Horn LC, Neuhaus J, Constantinides C, Liatsikos EN. Anatomical landmarks of radical prostatecomy. Eur Urol. 2007; 51:629–639.
40. Montorsi F, Wilson TG, Rosen RC, Ahlering TE, Artibani W, Carroll PR, et al. Best practices in robot-assisted radical prostatectomy: recommendations of the Pasadena Consensus Panel. Eur Urol. 2012; 62:368–381.
41. Alemozaffar M, Duclos A, Hevelone ND, Lipsitz SR, Borza T, Yu HY, et al. Technical refinement and learning curve for attenuating neurapraxia during robotic-assisted radical prostatectomy to improve sexual function. Eur Urol. 2012; 61:1222–1228.
42. Patel VR, Coelho RF, Chauhan S, Orvieto MA, Palmer KJ, Rocco B, et al. Continence, potency and oncological outcomes after robotic-assisted radical prostatectomy: early trifecta results of a high-volume surgeon. BJU Int. 2010; 106:696–702.
43. Menon M, Shrivastava A, Bhandari M, Satyanarayana R, Siva S, Agarwal PK. Vattikuti Institute prostatectomy: technical modifications in 2009. Eur Urol. 2009; 56:89–96.
44. Kumar A, Tandon S, Samavedi S, Mouraviev V, Bates AS, Patel VR. Current status of various neurovascular bundle-sparing techniques in robot-assisted radical prostatectomy. J Robot Surg. 2016; 10:187–200.
45. Tewari AK, Srivastava A, Huang MW, Robinson BD, Shevchuk MM, Durand M, et al. Anatomical grades of nerve sparing: a risk-stratified approach to neural-hammock sparing during robot-assisted radical prostatectomy (RARP). BJU Int. 2011; 108(6 Pt 2):984–992.
46. Inoue S, Shiina H, Hiraoka T, Mitsui Y, Sumura M, Urakami S, et al. Retrospective analysis of the distance between the neurovascular bundle and prostate cancer foci in radical prostatectomy specimens: its clinical implication in nerve-sparing surgery. BJU Int. 2009; 104:1085–1090.
47. Ko YH, Coelho RF, Sivaraman A, Schatloff O, Chauhan S, Abdul-Muhsin HM, et al. Retrograde versus antegrade nerve sparing during robot-assisted radical prostatectomy: which is better for achieving early functional recovery? Eur Urol. 2013; 63:169–177.
48. Kaul S, Savera A, Badani K, Fumo M, Bhandari A, Menon M. Functional outcomes and oncological efficacy of Vattikuti Institute prostatectomy with Veil of Aphrodite nerve-sparing: an analysis of 154 consecutive patients. BJU Int. 2006; 97:467–472.
49. Chien GW, Mikhail AA, Orvieto MA, Zagaja GP, Sokoloff MH, Brendler CB, et al. Modified clipless antegrade nerve preservation in robotic-assisted laparoscopic radical prostatectomy with validated sexual function evaluation. Urology. 2005; 66:419–423.
50. Badani KK, Shapiro EY, Berg WT, Kaufman S, Bergman A, Wambi C, et al. A pilot study of laparoscopic Doppler ultrasound probe to map arterial vascular flow within the neurovascular bundle during robot-assisted radical prostatectomy. Prostate Cancer. 2013; 2013:810715.
51. Ahlering TE, Eichel L, Chou D, Skarecky DW. Feasibility study for robotic radical prostatectomy cautery-free neurovascular bundle preservation. Urology. 2005; 65:994–997.
52. Finley DS, Osann K, Skarecky D, Ahlering TE. Hypothermic nerve-sparing radical prostatectomy: rationale, feasibility, and effect on early continence. Urology. 2009; 73:691–696.
53. Patel VR, Samavedi S, Bates AS, Kumar A, Coelho R, Rocco B, et al. Dehydrated human amnion/chorion membrane allograft nerve wrap around the prostatic neurovascular bundle accelerates early return to continence and potency following robot-assisted radical prostatectomy: propensity score-matched analysis. Eur Urol. 2015; 67:977–980.
54. Srivastava A, Chopra S, Pham A, Sooriakumaran P, Durand M, Chughtai B, et al. Effect of a risk-stratified grade of nerve-sparing technique on early return of continence after robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2013; 63:438–444.
55. Schatloff O, Chauhan S, Sivaraman A, Kameh D, Palmer KJ, Patel VR. Anatomic grading of nerve sparing during robotassisted radical prostatectomy. Eur Urol. 2012; 61:796–802.
56. Patel VR, Schatloff O, Chauhan S, Sivaraman A, Valero R, Coelho RF, et al. The role of the prostatic vasculature as a landmark for nerve sparing during robot-assisted radical prostatectomy. Eur Urol. 2012; 61:571–576.
57. Finley DS, Rodriguez E Jr, Skarecky DW, Ahlering TE. Quantitative and qualitative analysis of the recovery of potency after radical prostatectomy: effect of unilateral vs bilateral nerve sparing. BJU Int. 2009; 104:1484–1489.
58. Greco F, Hoda MR, Wagner S, Reichelt O, Inferrera A, Magno C, et al. Bilateral vs unilateral laparoscopic intrafascial nerve-sparing radical prostatectomy: evaluation of surgical and functional outcomes in 457 patients. BJU Int. 2011; 108:583–587.
59. Ficarra V, Novara G, Fracalanza S, D'Elia C, Secco S, Iafrate M, et al. A prospective, non-randomized trial comparing robot-assisted laparoscopic and retropubic radical prostatectomy in one European institution. BJU Int. 2009; 104:534–539.
60. Rocco B, Matei DV, Melegari S, Ospina JC, Mazzoleni F, Errico G, et al. Robotic vs open prostatectomy in a laparoscopically naive centre: a matched-pair analysis. BJU Int. 2009; 104:991–995.
61. Krambeck AE, DiMarco DS, Rangel LJ, Bergstralh EJ, Myers RP, Blute ML, et al. Radical prostatectomy for prostatic adenocarcinoma: a matched comparison of open retropubic and robot-assisted techniques. BJU Int. 2009; 103:448–453.
62. Patel VR, Sivaraman A, Coelho RF, Chauhan S, Palmer KJ, Orvieto MA, et al. Pentafecta: a new concept for reporting outcomes of robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2011; 59:702–707.
63. Kowalczyk KJ, Huang AC, Hevelone ND, Lipsitz SR, Yu HY, Ulmer WD, et al. Stepwise approach for nerve sparing without countertraction during robot-assisted radical prostatectomy: technique and outcomes. Eur Urol. 2011; 60:536–547.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download