Abstract
The practice of extended pelvic lymph node dissection (ePLND) remains one of the most controversial topics in the management of clinically localized prostate cancer. Although most urologists agree on its benefit for staging and prognostication, the role of the ePLND in cancer control continues to be debated. The increased perioperative morbidity makes it unpalatable, especially in patients with low likelihood of lymph node disease. With the advent of robotic assisted laparoscopic prostatectomy, many surgeons were slow to adopt ePLND in the robotic setting. In this study, we summarize the evidence for the prognostic and therapeutic roles of ePLND, review the clinical tools used for lymph node metastasis prediction and survey the numerous experiences of ePLND compiled by robotic urologic surgeons over the years.
Prostate cancer (PCa) is the second most common malignancy diagnosed and fifth leading cause of cancer death in men worldwide [1]. Death rates have been decreasing in the majority of developed countries, possibly related to improved treatment and/or early detection. Radical prostatectomy (RP) has proven effective in reducing mortality in patients with early PCa [2]. However, the role of the extended pelvic lymph node dissection (ePLND) at the time of RP lacks consensus and level 1 evidence.
With the advent of robot-assisted laparoscopic prostatectomy (RALP), ePLND has been demonstrated to be feasible and safe in the hands of experienced robotic surgeons [345678]. Patient selection remains critical for achieving therapeutic success. To that end, few advances have been made to supplement the time-tested PCa risk prediction nomograms in selecting patients with lymph node (LN) positive disease preoperatively. The role of the sentinel lymph node dissection (sLND) continues to evolve with the advent of new imaging technology. Although endorsed as an option by the European Association of Urology (EAU), sLND has been proven to be neither reliable in the perioperative nor the intraoperative setting.
Herein, we review the contemporary literature on the therapeutic effects of ePLND. Additionally, we will summarize strategies for patient selection, advancements in sLND, and the various techniques and experiences of robot-assisted ePLND in the literature.
Due to the lack of accurate preoperative imaging modality to assess nodal status, pelvic lymph node dissection (PLND) remains the gold standard for cancer staging. However, there is disagreement over the extent of dissection. This is chiefly due to the variant lymphatic drainage demonstrated for PCa. Early lymphangiography studies showed landing zones in the internal iliac, presacral and common iliac nodal regions [9]. The arrival of newer imaging technologies have provided an even more complex map, with additional drainage sites in the obturator, external iliac, pararectal and common iliac regions [101112].
As a result of the irregular drainage patterns, a standard template for PLND remains elusive. The limited PLND (lPLND) encompasses areas around the external iliac vessels and the obturator fossa. Early on, studies consistently demonstrated increased LN metastases as the template of PLND expanded [131415]. In fact, using an ePLND template that also included the common iliac vessels, internal iliac vessels, and the presacral area, Heidenreich et al. [14] showed that 42% of the LN metastases were found outside the lPLND template. A similar template was proposed by Mattei et al. [12] in a study combining single-photon emission computed tomography (SPECT) with template ePLND. The EAU guideline specifies a more limited template that includes nodes overlying the external iliac vessels, the obturator fossa, and the internal iliac artery [16], while noting that a more extended dissection to include the presacral nodes may reduce the number of patients with missed nodal metastases from 24% to 12% [17]. As a measure of quality control, autopsy series have suggested that removal of 20 nodes was necessary for adequate locoregional staging [18].
The identification of LN metastases by properly performed ePLND can aid in patient selection for adjuvant therapy after RP. There is strong, level I evidence for immediate adjuvant hormonal therapy for men with pelvic LN metastasis [1920]. In addition, retrospective data suggest adjuvant radiation therapy may benefit pN1 patients treated concurrently with androgen deprivation therapy, especially in those with oligometastatic disease [21].
Though most would agree on the benefit of ePLND for LN staging, the evidence for the therapeutic effect of ePLND is controversial. Schiavina et al. [22] demonstrated that regardless of nodal status, the removal of at least 10 LNs during RP led to improved biochemical progression free survival (bPFS). A study comparing RP patients receiving lPLND and ePLND by 2 different surgeons showed that the ePLND patients had superior 5-year bPFS of 30.1% vs. 7.1%, 10-year metastasis free survival of 62.2% vs. 22.2%, and 10-year cancer specific survival (CSS) of 83.6% vs. 52.6% [23]. A population study using data from surveillance, epidemiology, and end results program (SEER) corroborated with the finding that CSS was improved with more LNs removed [24]. This study suggested that lymphadenectomy is even beneficial in patients with pN0 disease as long as the number of nodes removed exceeded 10.
The explanation for the improvement in oncologic outcomes accompanying RP and PLND was scrutinized by several studies of specifically patients with pN1 disease. Within this high risk population, a divergence in cancer related outcomes emerged between patients with oligometastatic disease (1 positive LN) and those with heavier disease burdens. The oligometastatic group achieved far better bPFS (39% vs. 10%–14%), clinical progression free survival (cPFS) (54% vs. 38%–40%), and CSS (92% vs. 64%–75%) [25]. The authors thus concluded that surgical extirpation has curative potential in the oligometastatic setting. Follow-up studies confirmed the dichotomy of CSS between patients with 1–2 metastatic pelvic LNs vs. those with heavier nodal disease burden [2627].
Furthermore, 10-year CSS in pN1 patients was affected by the number of LNs removed during lymphadenectomy, ranging from 74.7% to 97.9% as the number of removed nodes increased from 8 to 45 [28]. This increase in survival associated with higher LN yield may be partially explained by the oft described Will Rogers effect [29]. In this situation, ePLND will uncover otherwise occult metastatic LN disease and result in cancer upstaging. In turn, this leads to an uneven statistical comparison between the mistakenly understaged lPLND patients and the correctly upstaged ePLND patients. On the other hand, studies utilizing immunohistochemistry and polymerase chain reaction have demonstrated occult micrometastatic disease in 13%–17% of the LNs deemed negative by routine histologic examination [3031]. These patients may stand to benefit from the surgical excision of the micrometastatic LNs by way of ePLND.
On the contrary, the therapeutic efficacy of PLND was challenged by several studies conducted during the prostate-specific antigen (PSA) era. In a large retrospective study from the Mayo Clinic consisting of 7,036 patients undergoing RP from 1987 to 2000, the number of LNs removed did not correlate with bPFS, cPFS, or CSS [32]. In a study restricted to low risk PCa patients, it was shown that the performance of lPLND did not lead to higher bPFS [33]. This was corroborated by a population based study using the CaPSURE database [34]. These studies fundamentally differed from the previously described studies supporting ePLND in that they included patients predominantly with low risk disease and low likelihood of LN metastases. Furthermore, all 3 studies reported inadequate LN yield according to the 20 node threshold proposed by Weingartner. In summary, these studies argue convincingly against the use of ePLND in patients with low risk PCa with low likelihood of LN metastases.
In the end, the therapeutic efficacy of ePLND can only be proven by a well-designed, prospective, randomized controlled trial. In 2012, Ji et al. [35] seemingly concluded the controversy with the results of such a study showing a benefit for ePLND on bPFS in patients with high and intermediate risk PCa. Unfortunately, the article was later retracted due to academic misconduct and data falsification. Briganti et al. [36] have recently outlined several important requirements for a meaningful study. The urologic community eagerly awaits the results.
As ePLND can be associated with considerable perioperative morbidity, the importance of appropriate patient selection cannot be overstated. While the benefit derived from risk prediction nomograms have been well documented, sLND remains a promising, but inconsistent method of nodal metastasis detection. Accurate prediction of nodal disease can serve to spare low risk PCa patients from suffering the morbidity associated with unnecessary surgery. In addition, understaging of nodal metastasis is avoided in high risk patients.
Prediction of pathological stage at RP is commonly performed using the ‘Partin tables’ [37]. These were first described based on preoperative parameters including serum PSA level, clinical stage and biopsy Gleason score. Recently, the Partin nomogram was updated using a contemporary cohort of 5,629 consecutive patients undergoing RP between 2006 and 2011 [38]. Biopsy Gleason 9–10 PCa was found to have significantly greater risk of LN metastasis than Gleason 8 (odds ratio, 3.2). Additionally, patients diagnosed with Gleason 3+3 or 3+4 disease have less than 2% risk for LN metastasis and thus may be spared PLND at the time of prostatectomy.
To enhance the prognostic accuracy for LN metastasis, integration of systematic sextant biopsies was proposed by Conrad et al. [39] in the form of the Hamburg algorithm. Using multivariate logistic regression and classification and regression tree analyses, the authors demonstrated that the amount and distribution of undifferentiated Gleason grade 4 and 5 cancer in the biopsies were the best predictors of lymphatic spread followed by PSA [40]. Naya and Babaian [41] improved upon the Hamburg algorithm by inspecting the number and location of positive biopsy cores on the ipsilateral side of LN metastasis, the highest Gleason score, and the percentage of tumor and maximum tumor length in each positive biopsy core. Their analysis revealed ≥4 positive cores with any Gleason grade 4 or 5, serum PSA≥15.0 ng/mL or the presence of dominant Gleason 4 or 5 were independent predictors of LN metastasis.
Most of the abovementioned nomograms, however, were formulated based on LN metastasis patterns described from lPLND. As LN metastases occur frequently outside the lPLND template [4243], many authors have pointed to the need to construct more accurate prognostic nomograms based on nodal metastasis data from ePLND. Briganti et al. [44] developed the first such nomogram based on pretreatment PSA, clinical stage and biopsy Gleason score from 602 patients who underwent RP and ePLND. The model achieved a predictive accuracy of 76.2% and was also validated in a subset of patients with 20 or more nodes removed. Recently, this nomogram was updated using a contemporary cohort of 588 patients with localized PCa undergoing ePLND during RP between 2006 and 2010 [45]. By adding the percentage of positive cores as a variable, the prediction accuracy was improved to 87.6%. Using this model, 65.5% of PCa patients would be spared ePLND at the expense of 1.5% missed LN invasion.
Since 1999 the role of sLND as part of staging for PCa has been investigated [46]. In the EAU guidelines, sLND is regarded as an option if a PCa patient has a greater than 7% risk of LN metastasis [47]. Several techniques for sLND have been investigated. Most series combine preoperative nuclear medicine imaging with intraoperative radiotracer detection, while some have investigated the use of near-infrared fluorescent dye indocyanine green. With these different techniques, most report successful identification of sentinel lymph nodes (SLNs), which often lie outside the bounds of the traditional eLND (Supplementary Table 1).
sLND has been proposed to play various roles in the staging of PCa patients. Measuring sLND detection against surgical pathology of nodal tissue extracted from PLND, Ponholzer et al. [48] reported a positive predictive value of over 90% for patients with intermediate or high risk PCa. Winter et al. [49] performed sLND in over 1,200 patients and found that 16% of patients with LN metastases would have otherwise been classified as low risk by predictive nomograms and thus would not have undergone PLND. In some reports, over 50% of the SLN lie outside of the PLND template [50], suggesting that sLND is necessary in addition to the standard dissection. Others advocate for SLN to serve as an indicator for LN metastasis, such that if the SLN is negative, no additional PLND is necessary [51].
The technique for sLND uses intraprostatic injection of radiotracer (99mTechnetitium labeled nanocolloid) under transrectal guidance, followed by imaging with planar views (anterior and posterior) combined with computed tomography (CT) between 1 and 3 hours thereafter [50]. SPECT/CT in comparison with planar imaging alone offers an advantage for detection of 98% of the SLNs [52]. Additionally, lymphotrophic nanoparticle enhanced magnetic resonance imaging in combination with diffusion weighted imaging may be a promising new technology to detect smaller (>2 mm) LN metastasis [5354].
The adequacy of the sensitivity associated with sLND has yet to be proven. One explanation is that the radiotracer may be blocked by micrometastases in the lymphatic channels, thus leading to a high false negative rate [49]. Despite studies showing relatively high CSS associated with sLND, many advocate its use as an adjunct to eLND [55].
After RALP using the Da Vinci robotic system (Intuitive Surgical, Sunnyvale, CA, USA) gained traction within the urologic community, many surgeons studied the feasibility of robotic PLND. Transperitoneal approach was often employed, with some surgeons reporting placement of ports slightly higher than in RALP without PLND [4]. Perioperative parameters including operative time, estimated blood loss, length of hospital stay, costs and perioperative complications were shown to be similar in RALP with and without PLND [5657].
Van der Poel et al. [57] showed that operative time for robotic assisted PLND decreased over the first 150 cases before reaching a plateau of 49 minutes. However, the learning curve stretched to 400 cases as the LN yield increased from 10 nodes in the initial 50 cases to 18 in cases number 351–400. Similar to open series, more nodal metastases were discovered as LN yield increased.
Early on, evidence from several single institutional and population based studies corroborated with the finding of the prolonged learning curve to perform PLND. Cooperberg et al. [58] described lower rate of PLND in RALP (31.8%) compared to open RP (ORP) (47.8%). In population based studies using data from SEER [59] and the Center for Medicare and Medicaid Services [60], PLND was performed 4–5 times less often with RALP than with ORP. A more recent study using the National Cancer Database involving 50,671 patients showed that even more than a decade after the adoption of robotic surgery, the rate of PLND in RALP (65.4%) still lagged behind ORP (81.2%). The study also identified prostatectomies performed at high volume, academic centers as more likely to incorporate PLND [61]. However, even among fellowship-trained, academic urologists, 19% reported different indications and extent associated with robotic and open prostatectomies [62].
In addition, the quality of the PLND performed with robotic assistance may be suboptimal compared to ORP. Many authors have reported lower LN yield from RALP when compared to ORP [5863], although some claimed that the lower yield was due to variations in surgeon practice rather than the modality of surgery [6364]. The clinical significance is underscored by the finding of higher number of metastatic LNs in ORP [5863656667]. Reassuringly, many of these studies found similar rates of complications after ORP and RALP, with very few incidences of vascular and neural injuries reported [586365]. Despite the difference demonstrated in LN yield, few authors have published survival data following RALP and ORP. Thus, it is difficult to discern whether the difference in nodal yield will ultimately lead to compromised CSS.
Surgeons' reluctance to perform PLND, the lower LN yield and the lower incidence of metastatic LN associated with RALP can partially be explained by the abundance of low risk patients opting for surgery in the PSA era. As noted by Touijer et al. [62], the extent of LN dissection has progressively decreased despite the lack of scientific evidence. The stage migration brought on by PSA screening lowered the number of patients with LN metastases. On the other hand, some speculate that surgeons early on in the learning curve of robotic surgery forego PLND to minimize complications, reduce surgical time and avoid converting to open surgery [63]. Alternatively, they may be avoiding performing prostatectomy robotically in the patients with the highest risk of LN metastases [58]. In summary, even more than a decade after the adoption of RALP, the associated LN yield continues to be lower than in ORP. Taken together with the evidence from literature on the benefit of thorough PLND, inadequate robotic nodal dissection may put patients at risk for understaging and worse oncologic outcomes.
To address this issue, many groups have reported on the feasibility and results of ePLND in the robotic setting. The boundaries of the described extended templates are varied, but in general consist of nodes over the hypogastric vessels, the obturator fossa, the external iliac vessels and over the common iliac vessels to the ureteric crossing [7686970] or aortic bifurcation [64] (Supplementary Table 2). The standard or limited template either consisted of the obturator fossa alone [7] or in conjunction with the external iliac nodes [64686970]. The standard PLND (stPLND) yielded between 5–18 nodes, compared to 13–24 in ePLND. Only one series reported non-significant difference between the nodal yield between stPLND and ePLND [69]. In all series, LN metastases were found more frequently with ePLND (10%–24.1%) than with stPLND (0%–5.2%). This is especially important in those series that did not perform ePLND or stPLND according to preoperative cancer risk [76468], suggesting that significant understaging of LN status may occur with stPLND.
Sagalovich et al. [70] analyzed the rate of LN metastasis detection using a cutoff LN yield of 13. They found that in the patients with at least 13 nodes dissected, LN metastasis rate (21%) was far higher than in the patients with less than 13 nodes dissected (5.1%). This corroborated with findings in open prostatectomy series in which the detection of LN invasion was near zero with less than 10 nodes removed, rose sharply with 10–20 LN s removed, and plateaued at ≥28 nodes [71]. The authors thus proposed that at least 13 nodes should be removed during PLND of high risk PCa patients.
Two reports investigated the location of metastatic LNs. Jung et al. [68] found that 25% of the metastatic LNs were located in the internal iliac and common iliac regions. Furthermore, the internal iliac nodes may be a primary landing zone as 3 of 4 patients had exclusively internal iliac node metastasis. Similarly, Katz et al. [64] found that 2 of the 5 patients in their series with invasive pelvic LNs had exclusively LN metastases in the common iliac packet. Together, these findings suggest that common iliac and internal iliac nodes have the potential to harbor metastases and dissection in these areas must be carried out. Due to the complexity of prostate lymphatic drainage, ePLND is essential for accurate nodal staging.
No major vascular or neural injuries were reported in any of the robotic ePLND series. Analysis of robotic assisted ePLND and stPLND results indicated that symptomatic lymphoceles occurred at a similar rate in the two procedures. In addition, Sagalovich et al. [70] proved that postoperative continence and potency are not impaired due to ePLND as dissection takes place far away from the parasympathetic nervous plexus.
The low rate of complication associated with robotic assisted ePLND is in contrast to the findings in ORP series. Musch et al. [72] demonstrated that the incidence of symptomatic lymphocele was related to the extent of lymphadenectomy as well as the number of LNs removed. There were more than twice as many overall complications and lymphoceles following ePLND (19.8% and 10.3%) than lPLND (8.2% and 4.6%), leading to longer hospital stay in the ePLND patients [73]. In a prospective, randomized study in which ePLND and lPLND were performed on different sides of the pelvis in patients undergoing prostatectomy, Clark et al. [74] found complications attributable to node dissection occurring 3 times more often on the side of the ePLND. Strategies to combat the frequent occurrence of pelvic lymphoceles included avoiding dissection of lymphatics lateral to the external iliac artery, ligating the distal ends of lymphatics, placing 2 drains on either side of the pelvis, leaving drains until output is less than 50 mL per day, and avoiding injection of deep vein thrombosis prophylactic agents into the lower extremities [75].
In contrast, clinically significant lymphocele consistently occurred in approximately 3% of patients undergoing robotic assisted ePLND (Supplementary Table 2). In a study with CT scans obtained 6–12 weeks postoperatively, Orvieto et al. [76] found lymphoceles in 51% of the patients. However, only 2.6% of the patients required intervention. The lower incidence of lymphocele and other complications associated with robotic assisted ePLND may be due to the enhanced visualization during robotic surgery. Moreover, as most robotic prostatectomies are performed transperitoneally, small lymph leaks may be resolved via peritoneal absorption. The increased intraperitoneal pressure during robotic surgery may also help seal small lymphatic leaks. Additional studies are needed to further explore these hypotheses.
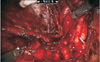
Previously, we have described a novel technique taking advantage of an intact urachus to enhance access to the deep hypogastric nodes [5]. The peritoneal incision was made just lateral to the medial umbilical ligament, leaving the urachal insertion intact. The third arm can then be used for medial and upward retraction to facilitate access to the hypogastric space (Fig. 1). After freeing up the tissue overlying the common iliac and internal iliac arteries to the takeoff of the obliterated umbilical artery, a plane in the perivesical fat is dissected medial to the internal iliac artery. This plane is dissected distally to the endopelvic fascia and anterolaterally to the obturator fossa. Attention needs to be paid to areas between the hypogastric subbranches, the subobturator nerve space, and along the superior path of the hypogastric artery on the second pass as nodal tissue may remain intact in these areas (Fig. 2). Finally, the nodal tissue over the external iliac/obturator fossa was dissected along with tissue over the Triangle of Marcille, which was accessed by retracting the external iliac vein medially (Fig. 3). Fig. 4 shows a completed right template.
With this technique, our median nodal yield was 22 nodes for primary surgeries and 21 for high risk patients following neoadjuvant systemic therapy. The percentage of cases with LN invasion was 16.4% for primary and 29% for neoadjuvant. Consistent with other reports [6468], hypogastric LNs were involved in 75% of pN1 primary cases and uniquely positive in 33%. The median time associated with each side of ePLND by the attending surgeon was 16 minutes compared to 25 minutes for trainees. Major complication (≥Clavien-Dindo classification grade III) rate was 3%, while only 1.8% of patients suffered symptomatic lymphocele.
Given the abundance of evidence for the prognostic and therapeutic values of ePLND, robotic surgeons face an alarming need to increase their practice of robotic assisted ePLND in the appropriately selected patients. Although technically challenging, many groups have demonstrated the feasibility and safety of robotic assisted ePLND. In addition, nodal yield and the detection rate of pelvic LN metastasis have equaled those in ORP series.
Figures and Tables
Fig. 1
For a left side extended pelvic lymph node dissection, the space of Retzius is opened and the urachus left intact to facilitate exposure of the hypogastric planes. The third arm grasper (top) will push the urachus up and contralaterally to facilitate exposure. Scan this QR code to see the accompanying video, or visit http://www.icurology.org or https://youtu.be/4Pyz8FBB7Jg.

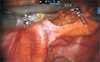
Fig. 2
The hypogastric (HG) identified from the takeoff from the external iliac (Ex Il) and cleared medially above and under the obliterated (OB) branch including the sub-branches headed medially towards the bladder. These areas often have to come out separately from the larger en bloc nodes from the iliac/obturator spaces. Scan this QR code to see the accompanying video, or visit http://www.icurology.org or https://youtu.be/vsLNewh99-o.

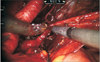
Fig. 3
The Triangle Marcille is an important space to clear. It can be accessed between the external iliac artery and vein (A) or lateral to the external iliac artery (B). The obturator nerve (B; at tip of scissors) should be identified and the surround lymphatics become the proximal extent of dissection. Scan this QR code to see the accompanying video, or visit http://www.icurology.org or https://youtu.be/OTGMRWOb1W8.


SUPPLEMENTARY MATERIALS
Supplementary Tables 1 and 2 can be found via http://www.icurology.org/src/sm/icurology-57-155-s001.pdf or below QR code scan. Supplementary Table 1. Studies of sentinel lymph node dissection. Supplementary Table 2. Studies of Robotic ePLND vs. s/l PLND. Accompanying videos can be found in the ‘Urology in Motion’ section of the journal homepage (http://www.icurology.org). The supplementary video clips can also be accessed by scanning a QR code located on the figures of this article, or be available on YouTube: video clip 1, https://youtu.be/4Pyz8FBB7Jg; video clip 2, https://youtu.be/vsLNewh99-o; video clip 3, https://youtu.be/OTGMRWOb1W8.

References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.
2. Bill-Axelson A, Holmberg L, Garmo H, Rider JR, Taari K, Busch C, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014; 370:932–942.
3. Davis JW, Shah JB, Achim M. Robot-assisted extended pelvic lymph node dissection (PLND) at the time of radical prostatectomy (RP): a video-based illustration of technique, results, and unmet patient selection needs. BJU Int. 2011; 108(6 Pt 2):993–998.
4. Feicke A, Baumgartner M, Talimi S, Schmid DM, Seifert HH, Müntener M, et al. Robotic-assisted laparoscopic extended pelvic lymph node dissection for prostate cancer: surgical technique and experience with the first 99 cases. Eur Urol. 2009; 55:876–883.
5. Williams SB, Bozkurt Y, Achim M, Achim G, Davis JW. Sequencing robot-assisted extended pelvic lymph node dissection prior to radical prostatectomy: a step-by-step guide to exposure and efficiency. BJU Int. 2016; 117:192–198.
6. Yee DS, Katz DJ, Godoy G, Nogueira L, Chong KT, Kaag M, et al. Extended pelvic lymph node dissection in robotic-assisted radical prostatectomy: surgical technique and initial experience. Urology. 2010; 75:1199–1204.
7. Yuh BE, Ruel NH, Mejia R, Novara G, Wilson TG. Standardized comparison of robot-assisted limited and extended pelvic lymphadenectomy for prostate cancer. BJU Int. 2013; 112:81–88.
8. Zorn KC, Katz MH, Bernstein A, Shikanov SA, Brendler CB, Zagaja GP, et al. Pelvic lymphadenectomy during robot-assisted radical prostatectomy: assessing nodal yield, perioperative outcomes, and complications. Urology. 2009; 74:296–302.
9. Raghavaiah NV, Jordan WP Jr. Prostatic lymphography. J Urol. 1979; 121:178–181.
10. Heesakkers RA, Jager GJ, Hövels AM, de Hoop B, van den Bosch HC, Raat F, et al. Prostate cancer: detection of lymph node metastases outside the routine surgical area with ferumoxtran-10-enhanced MR imaging. Radiology. 2009; 251:408–414.
11. Inoue S, Shiina H, Arichi N, Mitsui Y, Hiraoka T, Wake K, et al. Identification of lymphatic pathway involved in the spreading of prostate cancer by fluorescence navigation approach with intraoperatively injected indocyanine green. Can Urol Assoc J. 2011; 5:254–259.
12. Mattei A, Fuechsel FG, Bhatta Dhar N, Warncke SH, Thalmann GN, Krause T, et al. The template of the primary lymphatic landing sites of the prostate should be revisited: results of a multimodality mapping study. Eur Urol. 2008; 53:118–125.
13. Allaf ME, Palapattu GS, Trock BJ, Carter HB, Walsh PC. Anatomical extent of lymph node dissection: impact on men with clinically localized prostate cancer. J Urol. 2004; 172(5 Pt 1):1840–1844.
14. Heidenreich A, Varga Z, Von Knobloch R. Extended pelvic lymphadenectomy in patients undergoing radical prostatectomy: high incidence of lymph node metastasis. J Urol. 2002; 167:1681–1686.
15. Stone NN, Stock RG, Unger P. Laparoscopic pelvic lymph node dissection for prostate cancer: comparison of the extended and modified techniques. J Urol. 1997; 158:1891–1894.
16. Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014; 65:124–137.
17. Joniau S, Van den Bergh L, Lerut E, Deroose CM, Haustermans K, Oyen R, et al. Mapping of pelvic lymph node metastases in prostate cancer. Eur Urol. 2013; 63:450–458.
18. Weingärtner K, Ramaswamy A, Bittinger A, Gerharz EW, Vöge D, Riedmiller H. Anatomical basis for pelvic lymphadenectomy in prostate cancer: results of an autopsy study and implications for the clinic. J Urol. 1996; 156:1969–1971.
19. Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999; 341:1781–1788.
20. Messing EM, Manola J, Yao J, Kiernan M, Crawford D, Wilding G, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006; 7:472–479.
21. Abdollah F, Karnes RJ, Suardi N, Cozzarini C, Gandaglia G, Fossati N, et al. Impact of adjuvant radiotherapy on survival of patients with node-positive prostate cancer. J Clin Oncol. 2014; 32:3939–3947.
22. Schiavina R, Manferrari F, Garofalo M, Bertaccini A, Vagnoni V, Guidi M, et al. The extent of pelvic lymph node dissection correlates with the biochemical recurrence rate in patients with intermediate- and high-risk prostate cancer. BJU Int. 2011; 108:1262–1268.
23. Bivalacqua TJ, Pierorazio PM, Gorin MA, Allaf ME, Carter HB, Walsh PC. Anatomic extent of pelvic lymph node dissection: impact on long-term cancer-specific outcomes in men with positive lymph nodes at time of radical prostatectomy. Urology. 2013; 82:653–658.
24. Joslyn SA, Konety BR. Impact of extent of lymphadenectomy on survival after radical prostatectomy for prostate cancer. Urology. 2006; 68:121–125.
25. Bader P, Burkhard FC, Markwalder R, Studer UE. Disease progression and survival of patients with positive lymph nodes after radical prostatectomy. Is there a chance of cure? J Urol. 2003; 169:849–854.
26. Briganti A, Karnes JR, Da Pozzo LF, Cozzarini C, Gallina A, Suardi N, et al. Two positive nodes represent a significant cut-off value for cancer specific survival in patients with node positive prostate cancer. A new proposal based on a two-institution experience on 703 consecutive N+ patients treated with radical prostatectomy, extended pelvic lymph node dissection and adjuvant therapy. Eur Urol. 2009; 55:261–270.
27. Schumacher MC, Burkhard FC, Thalmann GN, Fleischmann A, Studer UE. Good outcome for patients with few lymph node metastases after radical retropubic prostatectomy. Eur Urol. 2008; 54:344–352.
28. Abdollah F, Gandaglia G, Suardi N, Capitanio U, Salonia A, Nini A, et al. More extensive pelvic lymph node dissection improves survival in patients with node-positive prostate cancer. Eur Urol. 2015; 67:212–219.
29. Albertsen PC, Hanley JA, Barrows GH, Penson DF, Kowalczyk PD, Sanders MM, et al. Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst. 2005; 97:1248–1253.
30. Ferrari AC, Stone NN, Kurek R, Mulligan E, McGregor R, Stock R, et al. Molecular load of pathologically occult metastases in pelvic lymph nodes is an independent prognostic marker of biochemical failure after localized prostate cancer treatment. J Clin Oncol. 2006; 24:3081–3088.
31. Pagliarulo V, Hawes D, Brands FH, Groshen S, Cai J, Stein JP, et al. Detection of occult lymph node metastases in locally advanced node-negative prostate cancer. J Clin Oncol. 2006; 24:2735–2742.
32. DiMarco DS, Zincke H, Sebo TJ, Slezak J, Bergstralh EJ, Blute ML. The extent of lymphadenectomy for pTXNO prostate cancer does not affect prostate cancer outcome in the prostate specific antigen era. J Urol. 2005; 173:1121–1125.
33. Weight CJ, Reuther AM, Gunn PW, Zippe CR, Dhar NB, Klein EA. Limited pelvic lymph node dissection does not improve biochemical relapse-free survival at 10 years after radical prostatectomy in patients with low-risk prostate cancer. Urology. 2008; 71:141–145.
34. Berglund RK, Sadetsky N, DuChane J, Carroll PR, Klein EA. Limited pelvic lymph node dissection at the time of radical prostatectomy does not affect 5-year failure rates for low, intermediate and high risk prostate cancer: results from CaPSURE. J Urol. 2007; 177:526–529.
35. Ji J, Yuan H, Wang L, Hou J. Is the impact of the extent of lymphadenectomy in radical prostatectomy related to the disease risk? A single center prospective study. J Surg Res. 2012; 178:779–784.
36. Briganti A, Giannarini G, Karnes RJ, Gandaglia G, Ficarra V, Montorsi F. What evidence do we need to support the use of extended pelvic lymph node dissection in prostate cancer? Eur Urol. 2015; 67:597–598.
37. Partin AW, Yoo J, Carter HB, Pearson JD, Chan DW, Epstein JI, et al. The use of prostate specific antigen, clinical stage and Gleason score to predict pathological stage in men with localized prostate cancer. J Urol. 1993; 150:110–114.
38. Eifler JB, Feng Z, Lin BM, Partin MT, Humphreys EB, Han M, et al. An updated prostate cancer staging nomogram (Partin tables) based on cases from 2006 to 2011. BJU Int. 2013; 111:22–29.
39. Conrad S, Graefen M, Pichlmeier U, Henke RP, Hammerer PG, Huland H. Systematic sextant biopsies improve preoperative prediction of pelvic lymph node metastases in patients with clinically localized prostatic carcinoma. J Urol. 1998; 159:2023–2029.
40. Lausen B, Sauerbrei W, Schumacher M. Classification and regression trees (CART) used for the exploration of prognostic factors measured on different scales. In : Dirschedl P, Ostermann R, editors. Conmputational statistics. Heidelberg: Physica-Verlag;1994. p. 483–496.
41. Naya Y, Babaian RJ. The predictors of pelvic lymph node metastasis at radical retropubic prostatectomy. J Urol. 2003; 170(6 Pt 1):2306–2310.
42. Heidenreich A, Varga Z, Von Knobloch R. Extended pelvic lymphadenectomy in patients undergoing radical prostatectomy: high incidence of lymph node metastasis. J Urol. 2002; 167:1681–1686.
43. Bader P, Burkhard FC, Markwalder R, Studer UE. Is a limited lymph node dissection an adequate staging procedure for prostate cancer? J Urol. 2002; 168:514–518.
44. Briganti A, Chun FK, Salonia A, Zanni G, Scattoni V, Valiquette L, et al. Validation of a nomogram predicting the probability of lymph node invasion among patients undergoing radical prostatectomy and an extended pelvic lymphadenectomy. Eur Urol. 2006; 49:1019–1026.
45. Briganti A, Larcher A, Abdollah F, Capitanio U, Gallina A, Suardi N, et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: the essential importance of percentage of positive cores. Eur Urol. 2012; 61:480–487.
46. Wawroschek F, Vogt H, Weckermann D, Wagner T, Harzmann R. The sentinel lymph node concept in prostate cancer: first results of gamma probe-guided sentinel lymph node identification. Eur Urol. 1999; 36:595–600.
47. Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011; 59:61–71.
48. Ponholzer A, Lamche M, Klitsch M, Kraischits N, Hiess M, Schenner M, et al. Sentinel lymphadenectomy compared to extended lymphadenectomy in men with prostate cancer undergoing prostatectomy. Anticancer Res. 2012; 32:1033–1036.
49. Winter A, Kneib T, Henke RP, Wawroschek F. Sentinel lymph node dissection in more than 1200 prostate cancer cases: rate and prediction of lymph node involvement depending on preoperative tumor characteristics. Int J Urol. 2014; 21:58–63.
50. Van den Bergh L, Joniau S, Haustermans K, Deroose CM, Isebaert S, Oyen R, et al. Reliability of sentinel node procedure for lymph node staging in prostate cancer patients at high risk for lymph node involvement. Acta Oncol. 2015; 54:896–902.
51. Wawroschek F, Vogt H, Wengenmair H, Weckermann D, Hamm M, Keil M, et al. Prostate lymphoscintigraphy and radio-guided surgery for sentinel lymph node identification in prostate cancer. Technique and results of the first 350 cases. Urol Int. 2003; 70:303–310.
52. Vermeeren L, Valdés Olmos RA, Meinhardt W, Bex A, van der Poel HG, Vogel WV, et al. Value of SPECT/CT for detection and anatomic localization of sentinel lymph nodes before laparoscopic sentinel node lymphadenectomy in prostate carcinoma. J Nucl Med. 2009; 50:865–870.
53. Thoeny HC, Triantafyllou M, Birkhaeuser FD, Froehlich JM, Tshering DW, Binser T, et al. Combined ultrasmall superparamagnetic particles of iron oxide-enhanced and diffusion-weighted magnetic resonance imaging reliably detect pelvic lymph node metastases in normal-sized nodes of bladder and prostate cancer patients. Eur Urol. 2009; 55:761–769.
54. Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003; 348:2491–2499.
55. Muck A, Langesberg C, Mugler M, Rahnenführer J, Wullich B, Schafhauser W. Clinical outcome of patients with lymph node-positive prostate cancer following radical prostatectomy and extended sentinel lymph node dissection. Urol Int. 2015; 94:296–306.
56. Atug F, Castle EP, Srivastav SK, Burgess SV, Thomas R, Davis R. Prospective evaluation of concomitant lymphadenectomy in robot-assisted radical prostatectomy: preliminary analysis of outcomes. J Endourol. 2006; 20:514–518.
57. van der Poel HG, de Blok W, Tillier C, van Muilekom E. Robot-assisted laparoscopic prostatectomy: nodal dissection results during the first 440 cases by two surgeons. J Endourol. 2012; 26:1618–1624.
58. Cooperberg MR, Kane CJ, Cowan JE, Carroll PR. Adequacy of lymphadenectomy among men undergoing robot-assisted laparoscopic radical prostatectomy. BJU Int. 2010; 105:88–92.
59. Feifer AH, Elkin EB, Lowrance WT, Denton B, Jacks L, Yee DS, et al. Temporal trends and predictors of pelvic lymph node dissection in open or minimally invasive radical prostatectomy. Cancer. 2011; 117:3933–3942.
60. Prasad SM, Keating NL, Wang Q, Pashos CL, Lipsitz S, Richie JP, et al. Variations in surgeon volume and use of pelvic lymph node dissection with open and minimally invasive radical prostatectomy. Urology. 2008; 72:647–652.
61. Wang EH, Yu JB, Gross CP, Smaldone MC, Shah ND, Trinh QD, et al. Variation in pelvic lymph node dissection among patients undergoing radical prostatectomy by hospital characteristics and surgical approach: results from the National Cancer Database. J Urol. 2015; 193:820–825.
62. Touijer KA, Ahallal Y, Guillonneau BD. Indications for and anatomical extent of pelvic lymph node dissection for prostate cancer: practice patterns of uro-oncologists in North America. Urol Oncol. 2013; 31:1517–1521.e1-2.
63. Silberstein JL, Vickers AJ, Power NE, Parra RO, Coleman JA, Pinochet R, et al. Pelvic lymph node dissection for patients with elevated risk of lymph node invasion during radical prostatectomy: comparison of open, laparoscopic and robot-assisted procedures. J Endourol. 2012; 26:748–753.
64. Katz DJ, Yee DS, Godoy G, Nogueira L, Chong KT, Coleman JA. Lymph node dissection during robotic-assisted laparoscopic prostatectomy: comparison of lymph node yield and clinical outcomes when including common iliac nodes with standard template dissection. BJU Int. 2010; 106:391–396.
65. Lallas CD, Pe ML, Thumar AB, Chandrasekar T, Lee FC, McCue P, et al. Comparison of lymph node yield in robot-assisted laparoscopic prostatectomy with that in open radical retropubic prostatectomy. BJU Int. 2011; 107:1136–1140.
66. Ledezma RA, Negron E, Razmaria AA, Dangle P, Eggener SE, Shalhav AL, et al. Robotic-assisted pelvic lymph node dissection for prostate cancer: frequency of nodal metastases and oncological outcomes. World J Urol. 2015; 33:1689–1694.
67. Truesdale MD, Lee DJ, Cheetham PJ, Hruby GW, Turk AT, Badani KK. Assessment of lymph node yield after pelvic lymph node dissection in men with prostate cancer: a comparison between robot-assisted radical prostatectomy and open radical prostatectomy in the modern era. J Endourol. 2010; 24:1055–1060.
68. Jung JH, Seo JW, Lim MS, Lee JW, Chung BH, Hong SJ, et al. Extended pelvic lymph node dissection including internal iliac packet should be performed during robot-assisted laparoscopic radical prostatectomy for high-risk prostate cancer. J Laparoendosc Adv Surg Tech A. 2012; 22:785–790.
69. Liss MA, Palazzi K, Stroup SP, Jabaji R, Raheem OA, Kane CJ. Outcomes and complications of pelvic lymph node dissection during robotic-assisted radical prostatectomy. World J Urol. 2013; 31:481–488.
70. Sagalovich D, Calaway A, Srivastava A, Sooriakumaran P, Tewari AK. Assessment of required nodal yield in a high risk cohort undergoing extended pelvic lymphadenectomy in robotic-assisted radical prostatectomy and its impact on functional outcomes. BJU Int. 2013; 111:85–94.
71. Briganti A, Chun FK, Salonia A, Gallina A, Zanni G, Scattoni V, et al. Critical assessment of ideal nodal yield at pelvic lymphadenectomy to accurately diagnose prostate cancer nodal metastasis in patients undergoing radical retropubic prostatectomy. Urology. 2007; 69:147–151.
72. Musch M, Klevecka V, Roggenbuck U, Kroepfl D. Complications of pelvic lymphadenectomy in 1,380 patients undergoing radical retropubic prostatectomy between 1993 and 2006. J Urol. 2008; 179:923–928.
73. Briganti A, Chun FK, Salonia A, Suardi N, Gallina A, Da Pozzo LF, et al. Complications and other surgical outcomes associated with extended pelvic lymphadenectomy in men with localized prostate cancer. Eur Urol. 2006; 50:1006–1013.
74. Clark T, Parekh DJ, Cookson MS, Chang SS, Smith ER Jr, Wells N, et al. Randomized prospective evaluation of extended versus limited lymph node dissection in patients with clinically localized prostate cancer. J Urol. 2003; 169:145–147.
75. Heidenreich A, Ohlmann CH, Polyakov S. Anatomical extent of pelvic lymphadenectomy in patients undergoing radical prostatectomy. Eur Urol. 2007; 52:29–37.
76. Orvieto MA, Coelho RF, Chauhan S, Palmer KJ, Rocco B, Patel VR. Incidence of lymphoceles after robot-assisted pelvic lymph node dissection. BJU Int. 2011; 108:1185–1190.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download