Abstract
The ability to accurately stage prostate cancer in both the primary and secondary staging setting can have a major impact on management. Until recently radiological staging has relied on computer tomography, magnetic resonance imaging, and nuclear bone scans to evaluate the extent of disease. However, the utility of these imaging technologies has been limited by their sensitivity and specificity especially in detecting early recurrence. Functional imaging using positron-emission tomography with a radiolabeled ligand targeted to prostate-specific membrane antigen has transformed the prostate cancer imaging landscape. Initial results suggest that it is a substantial improvement over conventional imaging in the setting of recurrence following primary therapy by having a superior ability to detect disease and to do so at an earlier stage. Additionally, it appears that the benefits seen in the secondary staging setting may also exist in the primary staging setting.
Prostate cancer (PCa) is the most commonly diagnosed malignancy and the second leading cause of cancer-related deaths amongst men [1]. Just as digital rectal examination, prostate-specific antigen (PSA) testing and transrectal biopsy have been stalwarts in the diagnosis of PCa, staging has relied on computed tomography (CT), magnetic resonance imaging (MRI) and technetium-99 m (99mTc)-methylene diphosphonate bone scans (BS). However, significant recent technological advancements have seen the staging landscape being transformed by positron-emission tomography (PET) based imaging. The principle of PET imaging is to visualise biochemical or physiological phenomena by utilising radiolabelled biomolecules that can be detected by the scanner as the tracer undergoes radioactive delay [2]. F-18 fluoro-deoxyglucose (FDG) is the most common tracer used in clinical practice but has been relatively disappointing when evaluating PCa because prostate cancers generally exhibit a low level of glucose metabolism [3]. As a result, a range of biomolecules and tracers were developed to improve PCa imaging of which a radiolabeled ligand targeted to prostate-specific membrane antigen (PSMA) has shown the greatest promise.
PSMA is a 750 amino acid type II transmembrane glycoprotein that is characterised by the monoclonal antibody 7E11.C5 [4]. It is highly prostate specific and is only expressed in limited healthy tissue in other parts of the body, primarily the salivary glands and proximal renal tubules [5]. Importantly, its uptake in noncancerous tissue, including benign prostatic tissue, is limited by a variety of physiological barriers, which makes it an ideal marker of PCa. While PSMA is expressed within the apical epithelium of the secretory ducts in benign prostatic tissue, studies have demonstrated that it up-regulates and migrates to the plasma membrane in malignant cells especially during the transition to hormone refractory disease [6]. Increased PSMA expression has been associated with higher tumour grade and increased risk of tumour progression [78]. Nonetheless, PSMA is expressed in neoplastic prostate tissue regardless of androgen status [9].
Identification of systemic disease at primary staging has the single greatest impact on prognosis and is of considerable importance in treatment planning [10]. While there has been substantial enthusiasm surrounding the use of 68PSMA-PET in evaluating patient with recurrence following primary treatment, recent studies have suggested that this new technology may also have a role in primary staging. The current standard of care involves imaging lymphatic and visceral spread with either CT or MRI and bone metastases with nuclear BS [11]. The utility of these conventional techniques are limited as reported in a large meta-analysis which found a pooled sensitivity and specificity of 42% and 82% respectively for CT in detecting nodal metastases. MRI performed even more poorly with identical specificity as CT but was only 39% sensitive [12]. Furthermore, the assessment of nodal involvement on conventional imaging is primarily based on size of lymph nodes rather than any functional or physiological information [13]. Thus, enter PET-based imaging, which has been shown to enhance detection of both nodal and osseous metastases in comparison to the aforementioned technologies.
In terms of nodal detection, Van Leeuwen et al. [14] compared primary staging PSMA-PET results to histopathology of thirty men with predominantly high-risk disease and reported a sensitivity and specificity of 64% and 95% respectively for PSMA-PET in the detection of lymph node metastases. These figures were further supported by a German study utilising PSMA-PET in the same setting [15]. The positive and negative predictive value of PSMA PET/CT was 88% and 82% respectively [14]. These rates are superior to those reported for imaging using 11C-choline or 18F-choline [1617]. In addition, the average size of lymph nodes that were missed on PSMA PET were 2.7 mm which is considerably smaller than the lower limit of the size of pathological nodes that are able to be detected on CT or MRI [1214]. This further compares favorably to 11C-choline PET/CT in which the mean size of false-negative lymph nodes was 4.2 mm [17]. Although the superiority over conventional imaging is evident, studies have suggested caution with the interpretation of PSMA-PET in this setting due to its limited sensitivity which has been variable between cohorts and thus reiterates the need for further research [18].
Perera et al. [19] reviewed the literature on PSMA in primary staging and identified seven studies (totaling 210 men) assessing use of PSMA in this setting, three of which reported lymph node detection rates with subsequent radical prostatectomy. Maurer et al. [15] presented the largest set of men (n=130) with PSMA detected lymph nodes who subsequently underwent pelvic lymphadenectomy. They reported a sensitivity of 65.9% and specificity of 98.9% for lymph nodes detected by PSMA-PET which is far superior to conventional imaging approaches. The specificity is particularly impressive and also reflects the quality of lymph node dissection in this unit: nodes identified by avidity on PSMA were almost always harvested by lymph node dissection.
With osseous metastases, similar to the advancements seen in the detection of nodal disease, functional PET scanning has had a comparable impact on staging. 99mTechnetium bone scanning has long been unchallenged in this landscape with reported sensitivities ranging from 62%–89% [20]. However, uptake of 99mTechnetium occurs in a number of nonmalignant bone processes including inflammation, degenerative joint disease and benign fractures leading to a low specificity. By contrast, 68Ga-PSMA PET scanning has shown promising sensitivity and specificity in detection of small volume skeletal disease in primary staging [19]. Pyka et al. [21] compared the diagnostic performance of bone scintigraphy and 68Ga-PSMA PET CT for skeletal staging in 126 men with PCa and reported that for those undergoing primary staging the sensitivity and specificity were 98.7% and 88.2% for PSMA-PET compared to 86.7% and 60.8% for bone scanning. They also noted that bone scanning rarely offers additional information to PSMA-PET, which can be used for detection of all metastatic sites as opposed to bone scanning alone.
Studies have also assessed the role of PSMA-PET in detection of visceral metastatic deposits, for example pulmonary or cerebral, but the evidence is still weak in this area mainly because of the lack of a histopathological correlation [22].
Currently, the primary application of PSMA-PET imaging appears to be in the biochemical recurrence (BCR) setting, that is, patients who have failed local therapy and are experiencing rise in their serum PSA levels but do not have metastatic disease on conventional imaging. The ability to accurately characterise the disease in the BCR setting as either local recurrence or metastatic spread is vital to choosing the optimal salvage therapy. In addition, having the capacity to make the aforementioned differentiation as early as possible is important in improving outcomes. This is exemplified in the case of salvage radiotherapy (SRT), which is optimally given at PSA levels <0.5 ng/mL [10]. King [23] highlighted the clinical impact of delivering SRT early by demonstrating a 2.6% loss of recurrence-free survival for each incremental 0.1 ng/mL PSA at the time of SRT.
One of the major pitfalls using conventional imaging in the BCR paradigm is its poor sensitivity in detecting early recurrence. Given that biochemical failure has been shown to precede clinical metastases by approximately 8 years, the utility of CT or BS at this early stage is poor [24]. Hence, most guidelines do not recommend performing conventional imaging when the PSA is less than 10 or PSA doubling time is greater than 10 months [25]. Previous studies have shown that there is a less than 5% likelihood of a positive BS unless serum PSA is above 40 and only a 3% chance if the PSA doubling time is greater than 6 months [2627]. Similarly, CT in the restaging setting performed equally poor with only 14% of men having a positive scan within 3 years of BCR and no positive scans when the PSA level was below 10 [2728]. The inability of these technologies to detect recurrent disease until a late stage has clinical implications as mentioned previously in the case of SRT.
68Ga-PSMA-PET has shown the potential to address some of the downsides of conventional imaging. In a large European study of 248 men with a median PSA of 1.99 ng/mL, 89.5% had a positive PSMA-PET/CT scan [29]. In a stark contrast to the results seen with conventional imaging, even men patients with a PSA level between 0.2–0.5 ng/mL displayed 57.9% detection rate. The improved sensitivity in this study can be credited to the superiority of PSMA-PET as it provided exclusive pathological findings and identified additional diseased regions in 32.7% and 24.6% of the cohort respectively. Similarly, an Australian series described that PSMA scan was positive in 46.3% when conventional imaging was negative [30]. Furthermore, in a comparison of PSMA-PET/CT to morphological imaging in patients with biochemical failure who have undergone salvage lymphadenectomy, Rauscher et al. [31] reported that PSMA scan had a 77.9% detection rate for lymph node metastases in histopathologically confirmed nodal disease compared to conventional imaging which had a rate of 26.9%. PSMA scans were also able to detect smaller positive nodes than older modalities (8.3 mm vs. 13.0 mm). Importantly, the additional information gained from performing a 68Ga-PSMA PET/CT had a significant clinical impact with management being changed in just under one-third of an Australian cohort [32].
Prior to 68Ga-PSMA, a variety of other tracers were utilised in PET-based imaging but they too have now been superseded. In a direct comparison with 18F-fluoromethylcholine in patients who have experienced biochemical failure, PSMA detected a significantly greater number of lesions and displayed a 37.5% higher detection rate when PSA values were below 0.5 ng/mL [33]. In the subset of patients for whom histological tissue was available for analysis, all the PSMA positive lesions were demonstrated to be true-positives under the microscope and none of the lesions that were 18F-choline PET positive and PSMA negative demonstrate PCa histologically. Moreover, in the same clinical setting, 68Ga-PSMA detected recurrence in 43.8% of the cohort who had previously had a negative 18F-Choline-PET/CT [34]. Furthermore, a Californian study which compared the utility of five different PET tracers (FDG, 11C-acetate, 11C- or 18F-choline, anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid and PSMA) in men with recurrent disease concluded that PSMA was superior to the others [35]. Overall, these initial studies suggest that 68Ga-PSMA is the PET tracer with the greatest potential.
Given that research regarding the utility of PSMA is still in its early stages, there is ongoing conjecture regarding the optimal clinical parameters to perform a 68Ga-PSMA PET scan. Ceci et al. [36] reported that absolute PSA level and PSA doubling time (PSAdt) were predictive factors of PET positivity and suggested optimal cutoff values of PSA 0.83 ng/mL and PSAdt 6.5 months. A recent meta-analysis displayed similar findings with a pooled PSMA positivity of 92% for PSAdt <6 months and 64% ≥6 months [19]. When analyzing absolute PSA values, the pooled estimate was 42% when PSA <0.2 ng/mL which increased to 95% with PSA values >2.00 ng/mL. While Eiber et al. [29] did not find PSAdt to be a predictor of disease detection, they rather suggested that PSA velocity was a significant factor for PSMA positivity with rates of 81.8%, 82.4%, 92.1%, and 100% in <1, 1 to <2, 2 to <5, and ≥5 ng/mL/y, respectively. Furthermore, the German study reported increased detection rates with cancers of Gleason grade 8 and above. Despite the need for further research in this area it is clear that parameters for which PSMA is able to detect disease is far superior to the other modalities currently available.
BCR after radiation therapy, as defined at the RTOG-ASTRO Phoenix Consensus Conference, consists of any PSA increase >2 ng/mL higher than the PSA nadir value, regardless of the serum concentration of the nadir and the short-term hormonal manipulation [37]. Currently, little or no evidence exists regarding the role of 68Ga-PSMA PET/CT in patients with BCR after having received radiotherapy (external beam or brachytherapy) as a primary treatment with curative intent. However, it is clear that 68Ga-PSMA PET/CT facilitates early detection and localisation of PCa recurrence—either local or distant—and that it has displayed superior sensitivity when compared to other staging modalities such as choline PET/CT [19].
In a German retrospective analysis in which 292 of the 319 included patients underwent a 68Ga-PSMA PET/CT post primary treatment, a prostatic lesion was seen in 22.5% of the cohort [38]. However, this subset of patients included those undergoing primary staging as well as patients who had received primary radiation therapy or androgen deprivation therapy (ADT). Histological testing with biopsy or surgery was only available in 42 of these patients and although lesion-based analysis allowed the authors to define 68Ga-PSMA PET/CT sensitivity (76%), specificity (100%), negative predictive value (91%), and positive predictive value (100%), they could not draw any separate conclusions for the group of patients that had previously received radiation therapy.
Similarly, Bluemel et al. [34] reported on 142 patients who had undergone primary treatment for PCa, of which 28 patients were treated initially with radiation therapy, and 56 with prostatectomy followed by SRT. Although the authors found that the highest rate of 68Ga-PSMA positivity was seen when PSA was >2 ng/mL, the heterogeneous cohort and small sample size did not allow any conclusions to be drawn regarding the primary radiotherapy group. This was also the case in small sample studies by Verburg et al. [39] and Morigi et al. [33] where only a small proportion of included patients had been primarily treated with radiotherapy. Furthermore, histopathological diagnosis was only available in a very limited amount of patients in these studies, if it was indeed available at all [40].
In the German study, 68Ga-PSMA PET/CT was more often positive in patients who were being treated with ADT, regardless of radiotherapy [38]. The authors put forward a number of hypotheses to explain this. Firstly, they argued patients undergoing 68Ga-PSMA PET/CT were more often patients with more advanced disease, and thus were more likely to be treated with ADT. Secondly, they stated it is probable that 68Ga-PSMA expression in tumours is differentially regulated by androgens. Conversely, Eiber et al. [29] reported no significant difference in detection efficacy with 68Ga-PSMA PET/CT, with regard to ADT.
It is not clear if the tumours visualised on 68Ga-PSMA PET/CT after radiotherapy, such as that seen in Fig. 1, are true relapses or if they reflect residual vitality in tumours after the patients have undergone radiation. Analysis of standardised uptake values has shown that 68Ga-PSMA uptake is high in primary tumours as well as in relapses or distant metastases. Higher contrast is however usually seen in lymph node metastases, followed by bone metastases, whereas local relapses and soft tissue metastases seem to present with lower 68Ga-PSMA uptake [38].
In summary, larger correlation studies with histological diagnosis will be necessary in order to clearly define the role of 68Ga-PSMA PET/CT in the subgroup of patients with primary radiation therapy failure. It should be noted that the current literature is limited by the significant heterogeneity of patients between studies, a small sample size, and often lack a histological confirmation of diagnosis.
The presence of PSMA expression in over 95% of all metastatic PCa cells allows for novel targeted radio-ligand theranostics. Currently, this uses the binding property of PSMA to Lutetium-177 (177Lu) as a radio-ligand therapy in metastatic castrate resistant prostate cancer (mCRPC).
There are four key papers, which analyze the safety and efficacy of 177Lu-PSMA radio-ligand therapeutics. These studies utilize PSMA-617 or 177Lu-DOTAGA (1,4,7,10-tetraaz acyclododececane, 1-(glutaric acid)-4,7,10-triacetic acid) as the principle-binding molecule. These ligands are selected for their lipophilicity, binding specificity, metabolic stability, as well as bio-distribution, and are superior to the PSMA antibody, which has cross selectivity for salivary gland and renal tissue potentially exposing these organs to unnecessary radiation effect [41].
Kratochwil et al. [42] from the University of Heidelberg reported on 30 patients with mCRPC treated with 177Lu-PSMA for one to three cycles. He noted 21 patients (70%) demonstrated a PSA response, of which this response was greater than 50% in 13 patients (40%). In addition, 73% of patients who completed three cycles of therapy sustained a PSA response of greater than 50% for over 24 months with measurable radiological decrease in metastasis size and volume.
Another German cohort demonstrated similar findings where 56 patients underwent 177Lu-PSMA therapy. 45 patients (80%) demonstrated a decrease in PSA levels and critically, follow-up was with 68Ga-PSMA PET which revealed partial remission in 5 patients, stable disease in 2 and progressive disease in 7 patients [43]. The median progression free survival was 13.7 months and severity of pain reduced in 2 of 6 patients (33%).
Rahbar et al. [44] reported on 2 studies, both conducted at the University Hospital Munster, Germany. The first reported on outcomes of 74 patients treated with a single dose of 177Lu-PSMA-617. PSA response was observed in 47 patients (64%) in whom a PSA decrease over 50% was seen in 23 patients (31%). Of note, with only one cycle of treatment, PSA remained stable in 35 patients (47%) and rose (greater than 25%) in 17 patients (23%). In a second study, Rahbar et al. [45] compared outcomes in those who received one cycle of treatment versus two in 28 patients. He noted that median survival was 29 weeks compared to 20 weeks based on historical published data. The clinical effectiveness of two treatments outweighed one, with PSA response demonstrated in 59% of patients after one treatment versus 75% after two treatments.
These studies report 177Lu-PSMA radioligand therapy as safe and effective in end stage progressive mCRPC in appropriately selected patients. Side effects including haematotoxicity, xerostomia, nausea, and fatigue were transient, short lived and uncommon. Whilst promising, these studies are small and have limited follow-up, all use PSA decline as their primary endpoint and only one utilized radiological criteria to define response. Further work is required to determine the optimal ligand, dose, timing and number of treatments. The role of 177Lu-PSMA radioligand therapy beyond the mCRPC remains to be evaluated.
As discussed above, accuracy in prediction of positive and negative results is important for a clinically useful staging tool. Perera et al. [19] systematically reviewed studies providing data correlating 68Ga-PSMA findings with histopathological-proven disease on a per-patient and perlesion basis. They identified five studies [1518404647] with a summary sensitivity of 80% and specificity of 97% for perlesion analysis and 86% for both sensitivity and specificity for per-patient analysis of 220 patients. They commented that the numbers were low and the confidence intervals wide but that the pooled data appeared to be promising compared to alternative imaging modalities for recurrent and metastatic PCa. In general, despite the heterogeneity between groups of studies assessing the accuracy of PSMA scanning in detection of PCa, on meta-analysis of studies divided into broad subtypes, 40% of scans were positive for patients undergoing primary staging for high risk disease and 76% were positive for those undergoing secondary staging for recurrent disease. The authors tested for predictors of positivity and found that PSA of around 0.2 to 0.3 ng/mL and PSAdt of <6 months were helpful thresholds for predicting a positive scan.
Overall, the initial published literature on 68Ga-PSMA PET/CT suggests that it represents a substantial improvement over conventional imaging for the staging of PCa. Despite this early promise, further research is required to validate its utility especially in the primary staging setting, whereas its role in the recurrent disease is becoming established. The ability to more accurately stage patients and detect recurrent disease at an earlier clinical time point has been shown to have a considerable impact on the optimal management of patients.
Figures and Tables
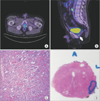 | Fig. 168Ga positron-emission tomography computed tomography scan of recurrent lesion 10 years after 74-Gy radical radiotherapy. (A) Axial reconstruction showing strong avid area in left peripheral zone. Further avidity evident but less intense on right side. (B) Sagittal reconstruction showing avid lesion in left peripheral zone prostate (arrow). Nonspecific avidity evident in kidney and bladder due to renal clearance of radio-ligand. (C) Haematoxylin and eosin (H&E, ×100 magnification) photomicrograph from transperineal biopsy targeting avid area confirmed recurrent prostate cancer, Gleason grade group 2 (Gleason 3+4). (D) Radical prostatectomy specimen megablock with area outlined showing cancer focus. |
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015; 65:5–29.
2. Griffeth LK. Use of PET/CT scanning in cancer patients: technical and practical considerations. Proc (Bayl Univ Med Cent). 2005; 18:321–330.
3. Jadvar H, Desai B, Ji L, Conti PS, Dorff TB, Groshen SG, et al. Prospective evaluation of 18F-NaF and 18F-FDG PET/CT in detection of occult metastatic disease in biochemical recurrence of prostate cancer. Clin Nucl Med. 2012; 37:637–643.
4. Murphy GP, Greene TG, Tino WT, Boynton AL, Holmes EH. Isolation and characterization of monoclonal antibodies specific for the extracellular domain of prostate specific membrane antigen. J Urol. 1998; 160(6 Pt 2):2396–2401.
5. Kiess AP, Banerjee SR, Mease RC, Rowe SP, Rao A, Foss CA, et al. Prostate-specific membrane antigen as a target for cancer imaging and therapy. Q J Nucl Med Mol Imaging. 2015; 59:241–268.
6. Santoni M, Scarpelli M, Mazzucchelli R, Lopez-Beltran A, Cheng L, Cascinu S, et al. Targeting prostate-specific membrane antigen for personalized therapies in prostate cancer: morphologic and molecular backgrounds and future promises. J Biol Regul Homeost Agents. 2014; 28:555–563.
7. Chang SS, Reuter VE, Heston WD, Gaudin PB. Comparison of anti-prostate-specific membrane antigen antibodies and other immunomarkers in metastatic prostate carcinoma. Urology. 2001; 57:1179–1183.
8. Ross JS, Sheehan CE, Fisher HA, Kaufman RP Jr, Kaur P, Gray K, et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res. 2003; 9:6357–6362.
9. Douglas TH, Connelly RR, McLeod DG, Erickson SJ, Barren R 3rd, Murphy GP. Effect of exogenous testosterone replacement on prostate-specific antigen and prostate-specific membrane antigen levels in hypogonadal men. J Surg Oncol. 1995; 59:246–250.
10. Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014; 65:467–479.
11. Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014; 65:124–137.
12. Hovels AM, Heesakkers RA, Adang EM, Jager GJ, Strum S, Hoogeveen YL, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008; 63:387–395.
13. Eiber M, Beer AJ, Holzapfel K, Tauber R, Ganter C, Weirich G, et al. Preliminary results for characterization of pelvic lymph nodes in patients with prostate cancer by diffusion-weighted MR-imaging. Invest Radiol. 2010; 45:15–23.
14. van Leeuwen PJ, Roobol MJ, Kranse R, Zappa M, Carlsson S, Bul M, et al. Towards an optimal interval for prostate cancer screening. Eur Urol. 2012; 61:171–176.
15. Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, et al. Diagnostic Efficacy of (68)Gallium-PSMA Positron Emission Tomography Compared to Conventional Imaging for Lymph Node Staging of 130 Consecutive Patients with Intermediate to High Risk Prostate Cancer. J Urol. 2016; 195:1436–1443.
16. Beheshti M, Imamovic L, Broinger G, Vali R, Waldenberger P, Stoiber F, et al. 18F choline PET/CT in the preoperative staging of prostate cancer in patients with intermediate or high risk of extracapsular disease: a prospective study of 130 patients. Radiology. 2010; 254:925–933.
17. Schiavina R, Scattoni V, Castellucci P, Picchio M, Corti B, Briganti A, et al. 11C-choline positron emission tomography/computerized tomography for preoperative lymph-node staging in intermediate-risk and high-risk prostate cancer: comparison with clinical staging nomograms. Eur Urol. 2008; 54:392–401.
18. Budäus L, Leyh-Bannurah SR, Salomon G, Michl U, Heinzer H, Huland H, et al. Initial experience of (68)Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. Eur Urol. 2016; 69:393–396.
19. Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, et al. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016; 06. 27. [Epub]. DOI: 10.1016/j.eururo.2016.06.021.
20. Daldrup-Link HE, Franzius C, Link TM, Laukamp D, Sciuk J, Jürgens H, et al. Whole-body MR imaging for detection of bone metastases in children and young adults: comparison with skeletal scintigraphy and FDG PET. AJR Am J Roentgenol. 2001; 177:229–236.
21. Pyka T, Okamoto S, Dahlbender M, Tauber R, Retz M, Heck M, et al. Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging. 2016; 43:2114–2121.
22. Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016; 13:226–235.
23. King CR. The timing of salvage radiotherapy after radical prostatectomy: a systematic review. Int J Radiat Oncol Biol Phys. 2012; 84:104–111.
24. Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999; 281:1591–1597.
25. Choueiri TK, Dreicer R, Paciorek A, Carroll PR, Konety B. A model that predicts the probability of positive imaging in prostate cancer cases with biochemical failure after initial definitive local therapy. J Urol. 2008; 179:906–910.
26. Cher ML, Bianco FJ Jr, Lam JS, Davis LP, Grignon DJ, Sakr WA, et al. Limited role of radionuclide bone scintigraphy in patients with prostate specific antigen elevations after radical prostatectomy. J Urol. 1998; 160:1387–1391.
27. Okotie OT, Aronson WJ, Wieder JA, Liao Y, Dorey F, DeKERNION JB, et al. Predictors of metastatic disease in men with biochemical failure following radical prostatectomy. J Urol. 2004; 171(6 Pt 1):2260–2264.
28. Kane CJ, Amling CL, Johnstone PA, Pak N, Lance RS, Thrasher JB, et al. Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology. 2003; 61:607–611.
29. Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of Hybrid 68Ga-PSMA Ligand PET/CT in 248 Patients with Biochemical Recurrence After Radical Prostatectomy. J Nucl Med. 2015; 56:668–674.
30. Shakespeare TP. Effect of prostate-specific membrane antigen positron emission tomography on the decision-making of radiation oncologists. Radiat Oncol. 2015; 10:233.
31. Rauscher I, Maurer T, Beer AJ, Graner FP, Haller B, Weirich G, et al. Value of 68Ga-PSMA HBED-CC PET for the assessment of lymph node metastases in prostate cancer patients with biochemical recurrence: comparison with histopathology after salvage lymphadenectomy. J Nucl Med. 2016; 57:1713–1719.
32. van Leeuwen PJ, Stricker P, Hruby G, Kneebone A, Ting F, Thompson B, et al. (68) Ga-PSMA has a high detection rate of prostate cancer recurrence outside the prostatic fossa in patients being considered for salvage radiation treatment. BJU Int. 2016; 117:732–739.
33. Morigi JJ, Stricker PD, van Leeuwen PJ, Tang R, Ho B, Nguyen Q, et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015; 56:1185–1190.
34. Bluemel C, Krebs M, Polat B, Linke F, Eiber M, Samnick S, et al. 68Ga-PSMA-PET/CT in patients with biochemical prostate cancer recurrence and negative 18F-Choline-PET/CT. Clin Nucl Med. 2016; 41:515–521.
35. Yu CY, Desai B, Ji L, Groshen S, Jadvar H. Comparative performance of PET tracers in biochemical recurrence of prostate cancer: a critical analysis of literature. Am J Nucl Med Mol Imaging. 2014; 4:580–601.
36. Ceci F, Uprimny C, Nilica B, Geraldo L, Kendler D, Kroiss A, et al. (68)Ga-PSMA PET/CT for restaging recurrent prostate cancer: which factors are associated with PET/CT detection rate? Eur J Nucl Med Mol Imaging. 2015; 42:1284–1294.
37. Roach M 3rd, Hanks G, Thames H Jr, Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006; 65:965–974.
38. Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015; 42:197–209.
39. Verburg FA, Pfister D, Heidenreich A, Vogg A, Drude NI, Vöö S, et al. Extent of disease in recurrent prostate cancer determined by [(68)Ga]PSMA-HBED-CC PET/CT in relation to PSA levels, PSA doubling time and Gleason score. Eur J Nucl Med Mol Imaging. 2016; 43:397–403.
40. Sahlmann CO, Meller B, Bouter C, Ritter CO, Ströbel P, Lotz J, et al. Biphasic 68Ga-PSMA-HBED-CC-PET/CT in patients with recurrent and high-risk prostate carcinoma. Eur J Nucl Med Mol Imaging. 2016; 43:898–905.
41. Weineisen M, Simecek J, Schottelius M, Schwaiger M, Wester HJ. Synthesis and preclinical evaluation of DOTAGA-conjugated PSMA ligands for functional imaging and endoradiotherapy of prostate cancer. EJNMMI Res. 2014; 4:63.
42. Kratochwil C, Giesel FL, Stefanova M, Benešová M, Bronzel M, Afshar-Oromieh A, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-Labeled PSMA-617. J Nucl Med. 2016; 57:1170–1176.
43. Baum RP, Kulkarni HR, Schuchardt C, Singh A, Wirtz M, Wiessalla S, et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: Safety and Efficacy. J Nucl Med. 2016; 57:1006–1013.
44. Rahbar K, Schmidt M, Heinzel A, Eppard E, Bode A, Yordanova A, et al. Response and tolerability of a single dose of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: a multicenter retrospective analysis. J Nucl Med. 2016; 57:1334–1338.
45. Rahbar K, Bode A, Weckesser M, Avramovic N, Claesener M, Stegger L, et al. Radioligand therapy with 177Lu-PSMA-617 as a novel therapeutic option in patients with metastatic castration resistant prostate cancer. Clin Nucl Med. 2016; 41:522–528.
46. Herlemann A, Wenter V, Kretschmer A, Thierfelder KM, Bartenstein P, Faber C, et al. 68Ga-PSMA positron emission tomography/computed tomography provides accurate staging of lymph node regions prior to lymph node dissection in patients with prostate cancer. Eur Urol. 2016; 01. 19. [Epub]. DOI: 10.1016/j.eururo.2015.12.051.
47. Pfister D, Porres D, Heidenreich A, Heidegger I, Knuechel R, Steib F, et al. Detection of recurrent prostate cancer lesions before salvage lymphadenectomy is more accurate with (68)Ga-PSMA-HBED-CC than with (18)F-Fluoroethylcholine PET/CT. Eur J Nucl Med Mol Imaging. 2016; 43:1410–1417.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download