Abstract
It is biologically plausible for dietary factors to influence bladder cancer risk considering that beneficial as well as harmful components of a diet are excreted through the urinary tract and in direct contact with the epithelium of the bladder. However, studies that investigated the association between dietary factors and bladder cancer (BC) risk have largely reported inconsistent results. The macronutrient intake and risk of BC could have yield inconsistent results across studies because of lack of details on the type, source and the quantities of different dietary fatty acids consumed. There is evidence to suggest that consumption of processed meat may increase BC risk. Dietary carbohydrate intake does not appear to be directly associated with BC risk. Even though a large number of studies have investigated the association between fruit/vegetable consumption/micronutrients in those and BC risk, they have yielded inconsistent results. Gender-specific subgroup analysis, details of how fruits and vegetables are consumed (raw vs. cooked), adequate control for smoking status/aggressiveness of the cancer and consideration of genetic make-up may clarify these inconsistent results. There is no strong evidence to suggest that supplementation with any common micronutrient is effective in reducing BC risk. These limitations in published research however do not totally eclipse the observation that a diet rich in fruits and vegetables and low in processed meat along with especially smoking cessation may convey some protective effects against BC risk.
Cancers in the bladder is the most common malignancy in the urinary tract and the age adjusted incidence rate is approximately nine per 10,000 for men and 2.2 for women globally [12]. Bladder cancer (BC) is the ninth most common cancer world-wide [2] and is the fifth most common cancer in the United States and represents 4.5% of all new cancer cases. In 2015, it is estimated that there will be 74,000 new cases of BC (56,320 in men and 17,680 in women) and an estimated 16,000 people will die of this disease (~11,510 men and 4,490 in women) [3].
Age, gender, cigarette smoking, exposures to aromatic amines, polycyclic aromatic hydrocarbons or arsenic, certain medications (phenacetin, cyclophosphamide, chlornaphazine), radiation and genetic factors are established risk factors for developing BC while there is suggestive evidence for several other factors, including diet [4]. Since BC is one of the most expensive diseases to treat because of the need for extended courses of treatment coupled with frequent follow-up examinations [5], examination of modifiable risk factors associated with this disease will aid in implementing preventive measures. Even though epidemiological evidence is inconsistent or controversial, diet is believed to be one of the most important modifiable risk factors for cancer prevention [6]. The purpose of this article is to critically review the findings published thus far on the associations between dietary factors and BC focusing on several aspects of diet, including, specific dietary factors such as food groups, intake and circulating concentrations of specific micronutrients.
Several case-control studies have documented that diets high in meat or fat is associated with higher risk of BC [7]. A limitation of these studies is that lack of detailed dietary data to examine the intake of different types of dietary fat or different sources of protein intake in relation to the risk of BC. A study that addressed these limitations showed that a 3% increase in the consumption of energy intake from animal protein was associated with a 15% higher risk of developing BC while a 2% increase in energy from plant protein intake was associated with a 23% lower risk of developing BC [8]. This study also documented that dietary intake of fat or carbohydrate was not associated with BC risk. However, other studies have documented statistically signif icant reduced odds of BC for high intakes of α-linolenic acid and vegetable fat and borderline statistically significant reduced odds for polyunsaturated fat and linoleic acid, indicating the importance of investigating the type, source and the quantities of different dietary fatty acids consumed [9]. Brinkman et al. [10] reported a statistically significant inverse association between olive oil intake and BC consistent with a linear dose-response relationship.
Even though some case-control studies have reported that a higher red meat intake was associated with BC risk [11], a meta-analysis published in 2014 based on 1,558,848 participants documented that red meat intake was not associated with BC, but suggested that high consumption of processed meat was correlated with rising risk of BC, especially in United States [12]. Similar results were observed in a study conducted in the New England region of the United States [13]. These studies indicate the importance of separating red meat and processed meat in evaluating BC risk in relation to meat consumption. Dietary carbohydrate intake does not appear to be associated with BC risk [9], but only limited data is available on this aspect. Two meta-analysis studies published in 2013, however, reported a positive association between diabetes mellitus and risk of BC, indirectly suggesting that control of carbohydrate intake may lower the BC risk [1415]. The studies associating macronutrients or macronutrient containing foods and risk of BC have been summarized in Table 1.
Even though a large number of studies have investigated the association between fruit and vegetable consumption and BC risk, they have yielded inconsistent results, especially with regard to the types of fruits and vegetables consumed. A male prospective cohort study documented that high cruciferous vegetable consumption may reduce BC risk, but other vegetables and fruits may not confer appreciable benefits against this disease [16]. A cohort of atomic-bomb survivors in Japan reported that consumption of green-yellow vegetables and fruits were protective against the development of BC [17]. A prospective cohort study of male smokers reported that consumption of fruits and vegetables, groups of fruits or vegetables (berries and cruciferous vegetables), specific fruits and vegetables or intakes of alpha-carotene, beta-carotene, lycopene, lutein/zeaxanthin, beta-cryptoxanthin, vitamins A, E, and C, and folate were not related to the risk of BC [18]. In contrast, a population-based case-control study conducted in non-Asians of Los Angeles, California showed a strong inverse associations between smoking-related BC risk and intake of dark-green vegetables, yellow-orange vegetables, citrus fruits/juices, tomato products, total carotenoids and vitamin C [19]. A similar inverse association between fruits and vegetables and risk of BC was observed in a meta-analysis [7]. However, a prospective population-based cohort study of Swedish women and men reported no statistically significant association between intakes of total fruits and vegetables, total fruits, total vegetables, citrus fruits, cruciferous vegetables, or green leafy vegetables and BC risk and these associations did not differ by sex or by smoking status [20]. A population-based case-control study performed in the Belgian province of Limburg showed that total fruit consumption, but not total vegetable intake reduces the effect of smoking on BC risk [21]. The European Prospective Investigation into Cancer and Nutrition (EPIC) study did not support an effect of fruit and vegetable consumption, combined or separately, on BC [22]. The EPIC study also documented that a variety in vegetable and fruit consumption also was not significantly associated with BC risk further providing evidence for the absence of any strong association between fruit and vegetable consumption and aggressive or nonaggressive BC risk [2324]. The latest publication from the EPIC study confirmed these observations [25]. A prospective analysis of older adults participating in the US Multiethnic Cohort Study reported that in women, total fruits and vegetables, total vegetables, yellow-orange vegetables, total fruits and citrus fruits were inversely associated with the risk of invasive BC. In addition, this study also reported that women with the highest intakes of vitamins A, C, and E; the carotenoids α-carotene, β-carotene, and β-cryptoxanthin; and folate had a lower risk of BC and for men, no associations for fruits, vegetables, or nutrients were found overall, although inverse associations were observed for vegetable intake among current smokers, and in ethnic-specific analyses, for fruit and vegetable intake among Latinos specifically. These findings suggest that higher consumption of fruits and vegetables may lower the risk of invasive BC among women and highlight the need for gender-specific subgroup analyses in future studies [27].
A hospital-based case-control study reported that consumption of raw, but not cooked cruciferous vegetables was inversely associated with bladder cancer risk [28]. A strong and significant inverse association was also observed between BC mortality and broccoli intake, in particular raw broccoli intake [29]. These studies suggest that cooking may alter the beneficial effects of protective nutrients and this may explain inconsistent results among different studies. In a population-based case-control study of bladder cancer in Maine, New Hampshire, and Vermont no association between fruits and vegetable consumption and risk of bladder cancer was observed [13]. Another US case-control study reported significant inverse associations between intakes of total vegetables, cruciferous vegetables, orange vegetables, dark green vegetables but not intakes of total fruits or citrus fruits and BC risk. Interestingly, this study also showed that the protective effect of vegetable consumption, especially cruciferous vegetables may be modified by genetic variants of GSTM1 and NAT2, suggesting that genetic makeup of individuals may also contribute to inconsistent results reported by different studies [27].
A dose-response meta-analysis of observational studies supported the hypothesis that intakes of fruit and vegetables may reduce the risk of BC [29]. An updated meta-analysis of observational studies published in 2015 also suggested that intake of vegetables and fruits may significantly reduce the risk of BC [30]. A systematic review and meta-analysis published in 2015 as part of the World Cancer Research/American Institute for Cancer Research Continuous Update Project, however, documented that current evidence from cohort studies is not consistent with a role for fruits and vegetables in preventing BC [31]. A preferred reporting items for systematic reviews and meta-analyses (PRISMA Compliant) study that included relevant prospective studies up to 2014 also documented that there is little evidence to support a beneficial effect for total fruits, vegetables or both together and citrus intake against BC [32]. The studies associating fruits and vegetables or micronutrients and risk of BC have been summarized in Table 2.
Studies have suggested a protective role of serum concentrations of selenium on BC risk, but not serum concentrations of retinol or beta-carotene [33]. However, a meta-analysis of epidemiological studies published in 2014 reported that higher serum or plasma concentrations of vitamin A, total carotenoids, α-carotene, β-carotene, lutein and zeaxanthin were associated with lower risk of BC [34]. A case-control study conducted by Liang et al. [35] suggested potential protective effect of plasma concentrations of alpha-tocopherol and retinol on BC risk. A large prospective study conducted among Japanese-American men documented that cigarette smoking, which is a strong risk factor for BC, may explain the apparent protective effect of individual and total carotenoids against this disease [36]. Other studies that investigated joint effect of plasma carotenoids and tobacco smoking suggested that BC may be a preventable disease through nutritional intervention, especially in smokers [37].
Some studies have reported that vitamin D status, as measured by serum 25-hydroxyvitamin D (25(OH) D) concentration, when it is low in male smokers had a nearly two-fold increased risk of BC compared to men with higher levels [38]. In contrast to this earlier report, the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Study observed no association between serum vitamin D status and risk of BC. It is possible that these differences between the 2 studies could be due to the inclusion of women and nonsmokers in the latter study population or due to the differences in the distribution of vitamin D concentrations between the two study populations [39]. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study provided additional support for an etiologic role for vitamin D in BC and suggested that free, rather than total, circulating vitamin D may be a more relevant exposure when examining BC risk in relation to vitamin D [40]. Other studies reported that individuals with low levels of plasma 25(OH)D(3) may be at high risk of more aggressive forms of BC, suggesting the importance of investigating the association by the aggressiveness of the disease [41]. A retrospective analysis of data in the Veterans Integrated Service Network-9 (southeastern US) suggested that adequate serum vitamin D levels early in the course of the disease provide the best opportunity to improve outcomes of BC [42]. Finally, a systemic review and meta-analysis published in 2015 documented that serum 25-hydroxyvitamin D concentrations were significantly associated with lower risk of BC [43]. The studies associating circulating concentrations of micronutrients and risk of BC have been summarized in Table 3.
US adults in the Cancer Prevention Study II cohort documented that regular use of vitamin E supplements (for ≥10 years), but not vitamin C was associated with a reduced risk of BC mortality [44]. The prospective Health Professionals Follow-Up Study, however, observed a significant dose-response relation not only for vitamin E but also for vitamin C supplement use and BC risk among US men [45]. In contrast, another study reported that the longterm use of commonly consumed supplemental vitamins (multivitamins, beta-carotene, retinol, folic acid, and vitamins B1, B3, B6, B12, C, D and E) have no significant effect on reducing BC risk [46]. The studies associating micronutrient supplements and risk of BC have been summarized in Table 4.
A limited number of studies have investigated other food components in relation to BC such as dairy, tea, egg and fish. A study conducted among Swedish men and women reported that the total dairy intake, intake of milk or cheese were not significantly associated with risk of BC, but a statistically significant inverse association was observed for the intake of cultured milk (sour milk and yogurt) [47]. However, The Netherlands Cohort Study on Diet and Cancer only provided weak evidence that BC risk is inversely associated with lower intake of fermented dairy products [48]. With regard to tea, Wang et al. [49] reported that green tea may have a protective effect on BC in Asians while Zhang et al. [50] observed that in Western countries, an increase in tea consumption in general may reduce the risk of BC. A meta-analysis published in 2013 reported that overall, there was no significant association between egg consumption and BC but, increased risk of bladder cancer was detected in North/South America and fried egg intake positively associated with BC risk [51]. The overall current literature on fish consumption and the risk of BC suggested no association. The studies associating other food items and risk of BC have been summarized in Table 5 [52].
In summary, studies that investigated the association between dietary factors and BC risk have largely reported inconsistent results. There is no strong evidence to suggest that specific dietary factors or supplementation with any common micronutrient present in food is effective in reducing BC risk. These limitations in published research however do not totally eclipse the observation that a diet rich in fruits and vegetables and low in processed meat along with especially smoking cessation may convey some protective effects against BC risk.
Figures and Tables
Table 1
Summary of the studies associating macronutrient and macronutrient containing foods and risk of bladder cancer

| Study | Dietary factor | Publication year | No. of patients | Study method | Recruitment/follow-up period | Main findings |
|---|---|---|---|---|---|---|
| Steinmaus et al. [7] | Fat | 2000 | 38 Articles, 4,578 cases | Meta-analysis | 1980–1999 | 37% Elevated risk of BC with high intakes of fat |
| Allen et al. [8] | Protein | 2013 | 469,339 Participants, 1,416 cases | Multicenter, prospective | 1992–2000 | 3% Increase in the consumption of energy intake from animal protein was associated with a 15% higher risk of developing BC while a 2% increase in energy from plant protein intake was associated with a 23% lower risk of developing BC |
| Brinkman et al. [9] | Fat | 2011 | 322 Cases and 239 controls | Case-control | 1998–2001 | 73% Reduced odds of BC with high intakes of α-linolenic acid |
| 61% Reduced odds of BC with high intakes of vegetable fat. Borderline statistically significant reduced odds were detected for polyunsaturated fat and linoleic acid | ||||||
| Brinkman et al. [10] | Olive oil | 2011 | 200 Cases and 386 controls | Case-control | 1999–2004 | Higher intake of olive oil was associated with 53% reduced risk of BC |
| Lin et al. [11] | Red meat | 2012 | 884 Cases and 878 controls | Case-control | 1999 | A significant dose response trend of elevated risk of developing BC with high intakes of red meat |
| Li et al. [12] | Red and Processed meat | 2014 | 1,558,848, 7,562 Cases | Meta-analysis | 1980–2012 | High consumption of processed meat but not red meat was correlated with rising risk of BC |
| Wu et al. [13] | Processed meat | 2012 | 1,068 Cases and 1,266 controls | Case-control (New England Bladder Cancer Study) | 2001–2004 | High intakes of processed meat and processed red meat were associated with 28% and 41% increased risk of BC |
Table 2
Summary of studies associating fruit and vegetable consumption and risk of bladder cancer
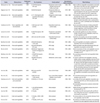
| Study | Dietary factor | Publication year | No. of patients | Study method | Recruitment/follow-up period | Main findings |
|---|---|---|---|---|---|---|
| Michaud et al. [16] | Fruits and vegetables | 1999 | 51,529 Participants, 252 cases | Prospective cohort study (Health Professionals Follow-Up Study) | 1986–1996 | High cruciferous vegetable consumption associated with reduce risk of BC, but other vegetables and fruits were not associated with risk of BC |
| Nagano et al. [17] | Fruits and vegetables | 2000 | 38,540 Participants | Prospective follow up study (Life Span Study Cohort) | 1979–1981 | Consumption of fruits and vegetables 2–4 times per week and almost every day was associated with 50% and 48% reduced risk of BC respectively |
| Michaud et al. [18] | Fruits and vegetables and micronutrients | 2002 | 27,111 Participants with complete information, 344 cases | Prospective cohort study (ATBC study) | 1985–1988 | Consumption of fruits and vegetables was not associated with the risk of BC |
| Dietary intakes of alpha-carotene, beta-carotene, lycopene, lutein/zeaxanthin, beta-cryptoxanthin, vitamins A, E, and C, and folate were not related to the risk of BC | ||||||
| Castelao et al. [19] | Fruits, vegetables and micronutrients | 2004 | 1,592 Cases and 1,592 controls | Population based case-control study | 1987–1996 | Inverse associations between smoking-related BC risk and intake of dark-green vegetables, yellow-orange vegetables, citrus fruits/juices, tomato products, total carotenoids and vitamin C |
| Larsson et al. [20] | Fruits and vegetables | 2008 | 82,002 Participants, 485 cases | Prospective-population based cohort study | 1987–1990 | No statistically significant association between intakes of total fruits and vegetables, total fruits, or total vegetables and BC |
| Kellen et al. [21] | Fruits and vegetables | 2005 | 200 Cases and 385 controls | Population-based case-control study | 1999–2004 | Total fruit consumption, but not total vegetable intake reduced the effect of smoking on BC risk by 39% |
| Buchner et al. [22] | Fruits and vegetables | 2009 | 478,533 Participants, 1,015 cases | EPIC study | 1991–2000 | Fruit and vegetable consumption combined or separately were not associated with BC |
| Buchner et al. [23] | Fruits and vegetables | 2011 | 452,185 Participants, 874 cases | EPIC study | 1991–2000 | Weak association was observed between fruit and vegetable consumption and risk of BC |
| Ros et al. [24] | Fruits and vegetables | 2012 | 468,656,421 Aggressive and 433 nonaggressive cases | EPIC study | 1991–2000 | Total consumption of fruits and vegetables was not associated with neither aggressive BC nor with nonaggressive BC |
| Bradbury et al. [25] | Fruits and vegetables | 2014 | ~470,000 Participants, 1,015 cases | EPIC study | 1992–2000 | No association between fruit and vegetable intake and risk of BC |
| Park et al. [26] | Fruits and vegetables | 2013 | 185,885 Participants, 581 cases | Multiethnic cohort study | 1991–2000 | In women, total fruits and vegetables, total vegetables, yellow-orange vegetables and citrus fruits were inversely associated with the risk of invasive BC. Women with the highest intakes of vitamins A, C, and E; the carotenoids α-carotene, β-carotene, and β-cryptoxanthin; and folate had a lower risk of BC. No associations between fruit and vegetable intake and risk of BC were observed in men |
| Lin et al. [28] | Fruits and vegetables | 2009 | 884 Cases and 878 controls | Case-control study | 1999–2009 | Highest vegetable consumption compared to lowest vegetable consumption was protective against BC |
| Wu et al. [13] | Fruits and vegetables | 2012 | 1,068 Cases and 1,266 controls | Case-control (New England Bladder Cancer Study) | 2001–2004 | No association between fruit and vegetable consumption and risk of BC |
| Steinmaus et al. [7] | Fruits and vegetables and carotenoids | 2000 | 38 Articles, 5,174 cases | Meta-analysis | 1980–1999 | Low intake of fruits and vegetables separately was associated with 40% and 16% elevated risk of BC, respectively |
| No association between low carotene/retinol diet and risk of BC | ||||||
| Yao et al. [29] | Fruits and vegetables | 2014 | 1,121,649 Participants, 12,610 cases | Meta-analysis | 1989–2013 | Intake of fruits and vegetables reduces the risk of developing BC by 17% |
| Liu et al. [30] | Fruits and vegetables | 2015 | 27 Studies | Meta-analysis | 1984–2014 | the relative risk of BC decreased by 8% and 9% for every 200 g/day increment in vegetable and fruit consumption, respectively |
| Vieira et al. [31] | Fruits and vegetables | 2013 | 15 Studies, 2,000–5,000 cases | Meta-analysis | 1988–2013 | No strong evidence of an association between fruits and vegetables and risk of BC |
| Xu et al. [32] | Fruits and vegetables | 2015 | 17 Studies, 9,447 cases | Meta-analysis | 1988–2014 | No evidence of nonlinear association was examined between fruits and vegetable intake and risk of BC |
Table 3
Summary of studies associating circulating concentrations of micronutrients and risk of bladder cancer

| Study | Dietary factor | Publication year | No. of patients | Study method | Recruitment/follow-up period | Main findings |
|---|---|---|---|---|---|---|
| Helzlsouer et al. [33] | Retinol and carotenoids | 1989 | 35 Cases and 70 matched controls | Case-control study | 1975–1986 | No association between circulating concentrations of retinol, retinol binding protein, or β-carotene and risk of BC |
| The risk of BC increased with decreasing levels of lycopene, α-tocopherol, and selenium | ||||||
| Tang et al. [34] | Vitamin A | 2014 | 25 Studies, 11,580 cases | Meta-analysis | 1988–2013 | Higher circulating concentrations of vitamin A , total carotenoids carotene, β-carotene, lutein and zeaxanthin were associated with lower risk of BC |
| Liang et al. [35] | Vitamin E and A | 2008 | 386 Cases and 389 controls | Case-control study | 1999 | 13% and 43% reduction in BC risk with increasing plasma a-tocopherol level and retinol levels respectively |
| Nomura et al. [36] | Carotenoids | 2003 | 109 Cases and 110 controls | Nested Case-control study | 1971–1977 | No statistically significant association between carotenoids and risk of BC after adjusting for smoking |
| Hung et al. [37] | Carotenoids | 2006 | 84 Cases and 173 controls | Case-control | 1993–1997 | Protective association between the circulating carotenoids and BC, Lower circulating concentrations of lutein and zeaxanthin were 5-6 times likely to increase risk of BC in smokers |
| Mondul et al. [38] | Vitamin D | 2010 | 250 Cases and 250 controls (males) | Nested case-control (ATBC study) | 1985–1988 | Lower serum vitamin D was associated with increased risk of BC |
| Mondul et al. [39] | Vitamin D | 2012 | 375 Cases and 375 controls | Nested Case-control study (PLCO study) | 1993–2006 | No statistically significant association between serum 25-hydroxyvitamin D and BC risk |
| Mondul et al. [40] | Vitamin D | 2012 | 250 Cases and 250 controls (males) | Nested Case-control (ATBC study) | 1985–1988 | Free vitamin D rather than total circulating vitamin D has a protective effect on risk of BC |
| Amaral et al. [41] | Vitamin D | 2012 | 1,125 Cases and 1,028 control | Case-control | 1998–2001 | Low levels of plasma 25(OH)D(3) may be at high risk of more aggressive forms of BC |
| Liao et al. [43] | Vitamin D | 2015 | 5 Eligible studies, 89,610 participants and 2,238 cases | Case-control | 1999–2008 | Inverse association between serum 25-hydroxyvitamin D level and risk of BC |
Table 4
Summary of studies associating micronutrient supplements and risk of BC

| Study | Dietary factor | Publication year | No. of patients | Study method | Recruitment/follow-up period | Main findings |
|---|---|---|---|---|---|---|
| Jacobs et al. [44] | Vitamin C and vitamin E | 2002 | 991,522 Participants | Cancer prevention study II | 1982–1998 | Regular use of vitamin E supplements (for ≥10 years), but not vitamin C was associated with a reduced risk of BC mortality |
| Michaud et al. [45] | Vitamin C, A and E | 2000 | 51,529 Participants | Health professionals follow-up study | 1986–1998 | Duration of vitamin E supplement intake was inversely associated with risk of BC; men with 10 or more years of vitamin E had a multivariate relative risk of 0.68 compared with never users. |
| Nonstatistically significant inverse associations were observed for supplemental dose of vitamins C and E among United States men | ||||||
| Hotaling et al. [46] | Vitamin and mineral supplements | 2011 | 77,050 Participants, 330 incident cases | Prospective follow-up study (VITAL cohort study) | 2000–2007 (6-year follow–up) | Long-term use of the vitamin, mineral supplements (multivitamins, beta-carotene, retinol, folic acid, and vitamins B1, B3, B6, B12, C, D and E) supplements not significantly associated with BC |
Table 5
Summary of studies associating other food items and risk of BC

| Study | Dietary factor | Publication year | No. of patients | Study method | Recruitment/follow-up period | Main findings |
|---|---|---|---|---|---|---|
| Larsson et al. [47] | Cultured milk, Yoghurt and dairy intake | 2008 | 82,002 Participants, 485 cases | Prospective follow-up study (Swedish Mammography Cohort and the Cohort of Swedish Men) | 1998–2007 | Women and men who consumed ≥2 servings of cultured milk per day had a 38% lower risk of bladder cancer than did those who never consumed cultured milk |
| Keszei et al. [48] | Dairy intake | 2010 | 1,549 Cases, 4,232 controls | Nested case-control study (Netherlands Cohort Study on Diet and Cancer) | 1986–2002 | Weak association between lower intake of fermented dairy products and risk of BC |
| Wang et al. [49] | Tea | 2013 | 17 Studies, 8,225 cases | Meta-analysis | 1980–2012 | No association between tea consumption and risk of BC |
| However, green tea consumption had a protective effect on BC | ||||||
| Zhang et al. [50] | Tea | 2015 | 57 Articles, 49,812 cases | Meta-analysis | 1980–2013 | No association between tea consumption and risk of BC |
| Li et al. [51] | Egg | 2013 | 13 Articles, 184,727 participants, 2,715 cases | Meta-analysis | 1988–2012 | No significant association was observed between egg consumption and BC |
| Egg consumption was associated with 40% elevated risk of BC in North and South America | ||||||
| Consumption of fried eggs increased relative risk of BC by 2 times | ||||||
| Li et al. [52] | Fish | 2011 | 14 Studies, 320,264 participants, 4,947 cases | Meta-analysis | 1986–2011 | No significant association between fish consumption and risk of BC |
References
1. Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Comperat E, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013; 64:639–653.
2. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386.
3. SEER stat fact sheets: bladder cancer [Internet]. Bethesda (MD): National Cancer Institute;2015. cited 2015 Dec 1. Avaiable from: http://seer.cancer.gov/statfacts/html/urinb.html.
4. Malats N, Real FX. Epidemiology of bladder cancer. Hematol Oncol Clin North Am. 2015; 29:177–189.
5. Busby JE, Kamat AM. Chemoprevention for bladder cancer. J Urol. 2006; 176:1914–1920.
6. Baena Ruiz R, Salinas Hernandez P. Diet and cancer: risk factors and epidemiological evidence. Maturitas. 2014; 77:202–208.
7. Steinmaus CM, Nuñez S, Smith AH. Diet and bladder cancer: a meta-analysis of six dietary variables. Am J Epidemiol. 2000; 151:693–702.
8. Allen NE, Appleby PN, Key TJ, Bueno-de-Mesquita HB, Ros MM, Kiemeney LA, et al. Macronutrient intake and risk of urothelial cell carcinoma in the European prospective investigation into cancer and nutrition. Int J Cancer. 2013; 132:635–644.
9. Brinkman MT, Karagas MR, Zens MS, Schned AR, Reulen RC, Zeegers MP. Intake of α-linolenic acid and other fatty acids in relation to the risk of bladder cancer: results from the New Hampshire case-control study. Br J Nutr. 2011; 106:1070–1077.
10. Brinkman MT, Buntinx F, Kellen E, Van Dongen MC, Dagnelie PC, Muls E, et al. Consumption of animal products, olive oil and dietary fat and results from the Belgian case-control study on bladder cancer risk. Eur J Cancer. 2011; 47:436–442.
11. Lin J, Forman MR, Wang J, Grossman HB, Chen M, Dinney CP, et al. Intake of red meat and heterocyclic amines, metabolic pathway genes and bladder cancer risk. Int J Cancer. 2012; 131:1892–1903.
12. Li F, An S, Hou L, Chen P, Lei C, Tan W. Red and processed meat intake and risk of bladder cancer: a meta-analysis. Int J Clin Exp Med. 2014; 7:2100–2110.
13. Wu JW, Cross AJ, Baris D, Ward MH, Karagas MR, Johnson A, et al. Dietary intake of meat, fruits, vegetables, and selective micronutrients and risk of bladder cancer in the New England region of the United States. Br J Cancer. 2012; 106:1891–1898.
14. Fang H, Yao B, Yan Y, Xu H, Liu Y, Tang H, et al. Diabetes mellitus increases the risk of bladder cancer: an updated metaanalysis of observational studies. Diabetes Technol Ther. 2013; 15:914–922.
15. Zhu Z, Zhang X, Shen Z, Zhong S, Wang X, Lu Y, et al. Diabetes mellitus and risk of bladder cancer: a meta-analysis of cohort studies. PLoS One. 2013; 8:e56662.
16. Michaud DS, Spiegelman D, Clinton SK, Rimm EB, Willett WC, Giovannucci EL. Fruit and vegetable intake and incidence of bladder cancer in a male prospective cohort. J Natl Cancer Inst. 1999; 91:605–613.
17. Nagano J, Kono S, Preston DL, Moriwaki H, Sharp GB, Koyama K, et al. Bladder-cancer incidence in relation to vegetable and fruit consumption: a prospective study of atomic-bomb survivors. Int J Cancer. 2000; 86:132–138.
18. Michaud DS, Pietinen P, Taylor PR, Virtanen M, Virtamo J, Albanes D. Intakes of fruits and vegetables, carotenoids and vitamins A, E, C in relation to the risk of bladder cancer in the ATBC cohort study. Br J Cancer. 2002; 87:960–965.
19. Castelao JE, Yuan JM, Gago-Dominguez M, Skipper PL, Tannenbaum SR, Chan KK, et al. Carotenoids/vitamin C and smoking-related bladder cancer. Int J Cancer. 2004; 110:417–423.
20. Larsson SC, Andersson SO, Johansson JE, Wolk A. Fruit and vegetable consumption and risk of bladder cancer: a prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2008; 17:2519–2522.
21. Kellen E, Zeegers M, Paulussen A, Van Dongen M, Buntinx F. Fruit consumption reduces the effect of smoking on bladder cancer risk. The Belgian case control study on bladder cancer. Int J Cancer. 2006; 118:2572–2578.
22. Buchner FL, Bueno-de-Mesquita HB, Ros MM, Kampman E, Egevad L, Overvad K, et al. Consumption of vegetables and fruit and the risk of bladder cancer in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2009; 125:2643–2651.
23. Buchner FL, Bueno-de-Mesquita HB, Ros MM, Kampman E, Egevad L, Overvad K, et al. Variety in vegetable and fruit consumption and risk of bladder cancer in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2011; 128:2971–2979.
24. Ros MM, Bueno-de-Mesquita HB, Kampman E, Büchner FL, Aben KK, Egevad L, et al. Fruit and vegetable consumption and risk of aggressive and non-aggressive urothelial cell carcinomas in the European Prospective Investigation into Cancer and Nutrition. Eur J Cancer. 2012; 48:3267–3277.
25. Bradbury KE, Appleby PN, Key TJ. Fruit, vegetable, and fiber intake in relation to cancer risk: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC). Am J Clin Nutr. 2014; 100:Suppl 1. 394S–398S.
26. Park SY, Ollberding NJ, Woolcott CG, Wilkens LR, Henderson BE, Kolonel LN. Fruit and vegetable intakes are associated with lower risk of bladder cancer among women in the Multiethnic Cohort Study. J Nutr. 2013; 143:1283–1292.
27. Tang L, Zirpoli GR, Guru K, Moysich KB, Zhang Y, Ambrosone CB, et al. Consumption of raw cruciferous vegetables is inversely associated with bladder cancer risk. Cancer Epidemiol Biomarkers Prev. 2008; 17:938–944.
28. Lin J, Kamat A, Gu J, Chen M, Dinney CP, Forman MR, et al. Dietary intake of vegetables and fruits and the modification effects of GSTM1 and NAT2 genotypes on bladder cancer risk. Cancer Epidemiol Biomarkers Prev. 2009; 18:2090–2097.
29. Yao B, Yan Y, Ye X, Fang H, Xu H, Liu Y, et al. Intake of fruit and vegetables and risk of bladder cancer: a dose-response meta-analysis of observational studies. Cancer Causes Control. 2014; 25:1645–1658.
30. Liu H, Wang XC, Hu GH, Guo ZF, Lai P, Xu L, et al. Fruit and vegetable consumption and risk of bladder cancer: an updated meta-analysis of observational studies. Eur J Cancer Prev. 2015; 24:508–516.
31. Vieira AR, Vingeliene S, Chan DS, Aune D, Abar L, Navarro Rosenblatt D, et al. Fruits, vegetables, and bladder cancer risk: a systematic review and meta-analysis. Cancer Med. 2015; 4:136–146.
32. Xu C, Zeng XT, Liu TZ, Zhang C, Yang ZH, Li S, et al. Fruits and vegetables intake and risk of bladder cancer: a PRISMA-compliant systematic review and dose-response meta-analysis of prospective cohort studies. Medicine (Baltimore). 2015; 94:e759.
33. Helzlsouer KJ, Comstock GW, Morris JS. Selenium, lycopene, alpha-tocopherol, beta-carotene, retinol, and subsequent bladder cancer. Cancer Res. 1989; 49:6144–6148.
34. Tang JE, Wang RJ, Zhong H, Yu B, Chen Y. Vitamin A and risk of bladder cancer: a meta-analysis of epidemiological studies. World J Surg Oncol. 2014; 12:130.
35. Liang D, Lin J, Grossman HB, Ma J, Wei B, Dinney CP, et al. Plasma vitamins E and A and risk of bladder cancer: a case-control analysis. Cancer Causes Control. 2008; 19:981–992.
36. Nomura AM, Lee J, Stemmermann GN, Franke AA. Serum vitamins and the subsequent risk of bladder cancer. J Urol. 2003; 170(4 Pt 1):1146–1150.
37. Hung RJ, Zhang ZF, Rao JY, Pantuck A, Reuter VE, Heber D, et al. Protective effects of plasma carotenoids on the risk of bladder cancer. J Urol. 2006; 176:1192–1197.
38. Mondul AM, Weinstein SJ, Mannisto S, Snyder K, Horst RL, Virtamo J, et al. Serum vitamin D and risk of bladder cancer. Cancer Res. 2010; 70:9218–9223.
39. Mondul AM, Weinstein SJ, Horst RL, Purdue M, Albanes D. Serum vitamin D and risk of bladder cancer in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening trial. Cancer Epidemiol Biomarkers Prev. 2012; 21:1222–1225.
40. Mondul AM, Weinstein SJ, Virtamo J, Albanes D. Influence of vitamin D binding protein on the association between circulating vitamin D and risk of bladder cancer. Br J Cancer. 2012; 107:1589–1594.
41. Amaral AF, Mendez-Pertuz M, Munoz A, Silverman DT, Allory Y, Kogevinas M, et al. Plasma 25-hydroxyvitamin D(3) and bladder cancer risk according to tumor stage and FGFR3 status: a mechanism-based epidemiological study. J Natl Cancer Inst. 2012; 104:1897–1904.
42. Peiris AN, Bailey BA, Manning T. Relationship of vitamin D monitoring and status to bladder cancer survival in veterans. South Med J. 2013; 106:126–130.
43. Liao Y, Huang JL, Qiu MX, Ma ZW. Impact of serum vitamin D level on risk of bladder cancer: a systemic review and meta-analysis. Tumour Biol. 2015; 36:1567–1572.
44. Jacobs EJ, Henion AK, Briggs PJ, Connell CJ, McCullough ML, Jonas CR, et al. Vitamin C and vitamin E supplement use and bladder cancer mortality in a large cohort of US men and women. Am J Epidemiol. 2002; 156:1002–1010.
45. Michaud DS, Spiegelman D, Clinton SK, Rimm EB, Willett WC, Giovannucci E. Prospective study of dietary supplements, macronutrients, micronutrients, and risk of bladder cancer in US men. Am J Epidemiol. 2000; 152:1145–1153.
46. Hotaling JM, Wright JL, Pocobelli G, Bhatti P, Porter MP, White E. Long-term use of supplemental vitamins and minerals does not reduce the risk of urothelial cell carcinoma of the bladder in the VITamins And Lifestyle study. J Urol. 2011; 185:1210–1215.
47. Larsson SC, Andersson SO, Johansson JE, Wolk A. Cultured milk, yogurt, and dairy intake in relation to bladder cancer risk in a prospective study of Swedish women and men. Am J Clin Nutr. 2008; 88:1083–1087.
48. Keszei AP, Schouten LJ, Goldbohm RA, van den Brandt PA. Dairy intake and the risk of bladder cancer in the Netherlands Cohort Study on Diet and Cancer. Am J Epidemiol. 2010; 171:436–446.
49. Wang X, Lin YW, Wang S, Wu J, Mao QQ, Zheng XY, et al. A meta-analysis of tea consumption and the risk of bladder cancer. Urol Int. 2013; 90:10–16.
50. Zhang YF, Xu Q, Lu J, Wang P, Zhang HW, Zhou L, et al. Tea consumption and the incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Eur J Cancer Prev. 2015; 24:353–362.
51. Li F, Zhou Y, Hu RT, Hou LN, Du YJ, Zhang XJ, et al. Egg consumption and risk of bladder cancer: a meta-analysis. Nutr Cancer. 2013; 65:538–546.
52. Li Z, Yu J, Miao Q, Sun S, Sun L, Yang H, et al. The association of fish consumption with bladder cancer risk: a meta-analysis. World J Surg Oncol. 2011; 9:107.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download