Abstract
Purpose
Laparoscopic urologists are familiar with both transperitoneal and retroperitoneal approaches. That experience is an advantage when devising a strategy for intra-abdominal lymph node biopsy. We report the feasibility and effectiveness of laparoscopic biopsy using a urological laparoscopic technique for the treatment of patients with clinically suspected intra-abdominal lymphoma.
Materials and Methods
From October 2010 to April 2015, a total of 22 patients underwent laparoscopic biopsy for suspected intra-abdominal lymphoma. We adopted a retroperitoneal approach for paraaortic or paracaval masses, whereas we used a transperitoneal approach for mesenteric, iliac, or obturator masses. Whenever possible, an entire node was removed; otherwise, the biopsy consisted of wedge resection sized at least 1 cm3.
Results
Biopsy specimens were obtained from the following lymph node sites: 10 paraaortic, 5 paracaval, 3 mesenteric, 2 obturator, 1 common iliac, and 1 perinephric fat. Laparoscopic lymph node biopsy was completed in all patients, and there were no conversions to open surgery. The median operating time was 97 minutes (range, 62–167 minutes). The estimated blood loss was <50 mL in all cases. Postoperatively, one patient (4.5%) had symptomatic chylous lymphocele that required surgical intervention. Precise diagnosis was established for all patients: malignant lymphoma in 20 patients and metastatic urothelial carcinoma and squamous cell carcinoma of unknown origin in 1 patient each. All lymphomas could be fully subclassified.
An accurate subgroup classification of lymphomas is mandatory because the classification influences prognosis as well as treatment [1]. The current World Health Organization classification for lymphomas is based on clinical information such as cytological, histological, and immunophenotypical features and other ancillary studies such as flow cytometry, fluorescence in situ hybridization, and genetic rearrangement testing [23]. Therefore, it is essential to obtain adequate tissue specimens for an accurate pathological diagnosis [4].
A patient presenting with an intra-abdominal lymphadenopathy is a common case scenario in clinical practice. In those who have associated peripheral lymphadenopathy, a biopsy can be performed under local anesthesia. In the absence of peripheral lymphadenopathy, however, a tissue specimen for diagnosis is more difficult to obtain [5].
Up to the 1980s, laparotomy was the only effective method for the diagnosis of intra-abdominal lymphoma. However, laparotomy is an invasive procedure associated with prolonged hospital stay and subsequent recovery time. In a series of 94 laparotomies for Hodgkin's lymphoma, Muskat et al. [6] reported that early and late (greater than 30 days) morbidity were 17% and 5%, respectively.
Image-guided percutaneous biopsies have been used because of their low morbidity and acceptable diagnostic yield, but they are often unsuccessful in yielding a definitive diagnosis with complete subclassification [78910]. Limitations are mostly related to the lack of adequate amount of tissue specimen for diagnosis.
Several studies have reported that laparoscopic intraabdominal biopsy is a safe and effective procedure for providing adequate biopsy sampling. In most previous reports, the procedure was performed by general surgeons using the transperitoneal approach [411121314]. However, a relatively high rate of conversion to laparotomy has been described, usually because of inadequate exposure, insufficient tissue, or postoperative adhesions. In fact, the transperitoneal approach sometimes seems somewhat troublesome, when the targeted retroperitoneal nodes are small in size or when excessive fatty tissue makes their identification difficult in obese patients [15]. A retroperitoneal approach that allows direct access to the proximity of large blood vessels seems more suitable in these cases.
Laparoscopic surgery in the urological field involves two different approaches, namely, transperitoneal or retroperitoneal. The availability of two approaches is a significant advantage when planning the strategy for intraabdominal lymph node biopsy.
Here, we report the feasibility and effectiveness of laparoscopic biopsy using the urological laparoscopic technique for the treatment of patients with clinically suspected intra-abdominal lymphoma.
From October 2010 to April 2015, a total of 22 patients underwent laparoscopic lymph node biopsy for suspected intra-abdominal lymphoma. With the approval of our Institutional Review Board, we retrospectively analyzed the data collected from these patients. All patients were referred to our institution from the Department of Hematology or Internal Medicine with imaging studies including routine computed tomography (CT) and fluorine-18 fluorodeoxyglucose positron emission tomography (18F-FDG PET). An excisional biopsy of peripheral lymph nodes was performed in two cases, but failed to establish the diagnosis. None of these patients underwent an imaging-guided biopsy of the abdominal mass prior to laparoscopy.
We adopted a retroperitoneal approach for paraaortic or paracaval masses, because this approach presents direct access to the target organ. A retroperitoneal space was created by using a preperitoneal distension balloon (Tyco Healthcare, Mansfield, MA, USA) in a complete lateral position. Conversely, we used a transperitoneal approach for mesenteric, iliac, or obturator masses. For mesenteric masses, a right semilateral position was used, permitting mobilization of the small intestine to the right. For obturator or iliac masses, a 10° Trendelenburg position was used to retract the bowels cephalad. In most patients, three ports were required. In some cases, placement of a fourth port was necessary to provide retraction and improve exposure.
Whenever possible, an entire node was removed and the base of the node was then clipped; otherwise, the biopsy consisted of wedge resection sized at least 1 cm3. After the biopsy, hemostasis at the cut edges of the lymph node was secured by electrocautery. In some cases, fibrin glue was used to prevent lymphocele formation or to control bleeding. The use of fibrin glue depended on the surgeons' preference. Specimens were sent for frozen and permanent sectioning by a pathologist. After confirming that an appropriate tissue specimen had been obtained, the surgery was completed. The tissue was divided as appropriate for routine, cytogenetic, and molecular studies. Molecular analyses that included immunoglobulin and T-cell receptor gene rearrangements and analysis of the Epstein-Barr virus genome were performed in some samples to confirm the diagnosis.
A total of 11 men and 11 women, ranging in age from 51 to 85 years, were included. One patient had been treated for a previous lymphoma and visited us with suspicion of recurrence. In the other 21 cases, abdominal enlargement was highly suggestive of primitive lymphoma. Biopsy specimens were obtained from the following lymph node sites: 10 paraaortic, 5 paracaval, 3 mesenteric, 2 obturator, 1 common iliac, and 1 perinephric fat. We determined the laparoscopic approach according to the lymph node location. Fifteen patients (9 paraaortic, 5 paracaval, and 1 perinephric fat) underwent surgery via the retroperitoneal approach (Fig. 1), whereas the remaining 7 patients (1 paraaortic, 3 mesenteric, 2 obturator, 1 common iliac) underwent surgery via the transperitoneal approach (Fig. 2).
All cases were completed laparoscopically. The median operating time was 97 minutes (range, 62–167 minutes). The estimated blood loss was <50 mL in all cases. The median postoperative length of stay was 4 days (range, 3–47 days). Patients who were not discharged from the hospital were generally transferred to the Department of Hematology within 5 days for further examination or initiation of treatment.
There were no periprocedural mortalities and we found only 1 important complication of symptomatic lymphocele. A 77-year-old woman developed abdominal fullness and pain on the 13th postoperative day after paraaortic lymph node biopsy. A CT scan revealed lymphocele in the retroperitoneal space, and a pigtail drainage tube was then placed, which revealed a large amount of serous milky fluid draining at the rate of 1,000 mL/day. After conservative management failed to stop the chylous lymphatic leakage, retroperitoneoscopic lymphostasis was performed on the 24th postoperative day. Fibrin glue was sprayed to pack the exposed paraaortic area until the obvious accumulation of lymphatic fluid disappeared. The drainage tube was finally removed 5 days after lymphostasis.
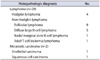
A precise diagnosis was obtained in all patients. Twenty patients were found to have malignant lymphoma and one each had metastatic urothelial carcinoma and squamous cell carcinoma of unknown origin. All lymphomas could be fully subclassified. The histopathological diagnosis for all patients is presented in detail in Table 1.
Urological laparoscopic surgery has evolved greatly since the introduction of laparoscopic nephrectomy in 1991 [16]. Today, various types of laparoscopic urological surgeries, such as laparoscopic adrenalectomy, radical nephrectomy, nephroureterectomy, radical prostatectomy, and radical cystectomy, are performed daily by an increasing number of urologists in hospitals and medical centers. Utilizing this experience, it is easy to approach mesenteric lymph nodes, retroperitoneal lymph nodes below the renal hilar region, or pelvic lymph nodes. With new advances in technology and instrumentation, some advanced laparoscopic procedures are currently available in high-volume centers with adequate laparoscopic expertise, such as extended pelvic lymph node dissection for bladder cancer [17] and retroperitoneal lymph node dissection for testicular cancer [18].
In the case of suspected intra-abdominal lymphoma, laparoscopy and image-guided percutaneous biopsy are two reasonable means to open laparotomy to take a tissue specimen. Currently, there are no evidence-based guidelines recommending one technique over the other [10]. Multiple studies have shown percutaneous biopsy to be an adequately sensitive technique for identifying the presence of lymphoma; however, it is often unsuccessful in yielding a definitive diagnosis with complete subgroup classification [78910]. In a series of 263 percutaneous image-guided biopsies used to diagnose suspected lymphoma, a diagnosis could be made in 90.1% of cases; however, only 75% of lymphomas could be fully subclassified [9]. In a study comparing laparoscopic biopsy with percutaneous biopsy for the diagnosis of abdominal lymphoma, significantly more patients in the laparoscopic biopsy group had sufficient tissue for ancillary studies when needed than in the percutaneous biopsy group: 95.5% and 68.2%, respectively [10]. In light of this evidence, it has been our policy to perform laparoscopic lymph node biopsy at first presentation in the case of isolated abdominal lymphadenopathy with suspicion of lymphoma.
In 1988, Salky et al. [19] described the first laparoscopic biopsy in a case of retroperitoneal disease. From the late 1990s to the early 2000s, three large case series were reported by general surgeons [1413]. The rate of conversion to open laparotomy, however, is relatively high (3.6%–17%), especially in the case of retroperitoneal lymph nodes. The primary reasons for conversion are previous surgery, obscure anatomy, inadequate explorations, inadequate sampling, and bleeding [4]. In these studies, the procedure was performed by using the transperitoneal approach. It may be possible to evade these causes of conversion by using a retroperitoneal approach. For mesenteric lymph nodes or interaortocaval lymph nodes, the transperitoneal approach may be safer and simpler than the retroperitoneal approach. There were no cases of conversion in our series. Although this was a small retrospective study, we consider that our study shows the feasibility of using two approaches appropriately in laparoscopic intra-abdominal lymph node biopsy. The ability to choose the operative approach according to circumstances represents a clear advantage for urologists.
One patient (4.5%) had a symptomatic chylous lymphocele, which was unsuccessfully managed by dietary intervention and needed surgical intervention. In this case, the retroperitoneal approach was applied for the paraaortic lymph node biopsy. Porte et al. [20] described surgical exploration of the retroperitoneal space through a direct vision endoscope without gas insufflation for retroperitoneal lymphadenopathy. Lymphorrhea persisting for more than 5 days was observed in 5 of the 118 patients. The retroperitoneal procedure may be associated with a high risk of lymphorrhea because there is no intraperitoneal space to absorb the lymphatic fluid. In 9 patients, we used fibrin glue to reduce postoperative lymphorrhea, but it was not used in this case. Several prospective studies have addressed the use of a fibrin sealant to reduce lymphadenectomy-related postoperative complications in patients undergoing dissection of the groin, axilla, or paraaortic lesions [2122232425]. However, these results remain controversial. Occurrence of these complications leads to a delay in the treatment of lymphoma. Therefore, the use of fibrin glue may be recommended in the retroperitoneal approach for intra-abdominal lymph node biopsy, especially when urgent chemotherapy is required for patients suspected to have high-grade lymphoma.
Previous laparoscopic biopsy experiences have shown a high frequency of false-negative diagnoses (0%–14%) [1411121314]. In our series, a precise diagnosis was established for all patients, and 20 lymphomas were completely subclassified. To minimize false-negative results, it is necessary to collect a sufficient quantity of a specimen tissue and to select an appropriate specimen as a target biopsy site. 18F-FDG PET is currently considered the standard modality for not only staging but also evaluating targeted biopsy sites [2627]. In an FDG-avid tumor, it is important to select a high FDG uptake lesion as a target site. Furthermore, when multiple lesions are present, it is important to decide from which lesion we can easily and safely perform a biopsy. A thorough CT and PET evaluation by the surgical team is mandatory prior to laparoscopy, so as to select the surgical approach and the route from which to approach the node.
Appropriate use of a transperitoneal or a retroperitoneal approach is safe and effective for laparoscopic lymph node biopsy in patients with suspected intra-abdominal lymphoma. Urologists should participate aggressively in this field, because these two approaches have become standard procedures in urological laparoscopy.
Figures and Tables
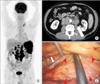 | Fig. 1During evaluation of unintended weight loss and night sweats, a 63-year-old male patient was found to have paraaortic lymph node swelling and an enlarged spleen. (A) Coronal section of fluorine-18 fluorodeoxyglucose positron emission tomography. The maximum standardized uptake values were 19.1 and 14.7 at the paraaortic lymph nodes and spleen, respectively. (B) Axial section on computed tomography. The retroperitoneal approach was used (arrow). (C) Laparoscopic image. The swollen lymph node is identifiable in front of the psoas muscle (arrowheads). The ureter is running in contiguity with the lymph node (arrow). The histopathological diagnosis was the nodular sclerosis subtype of classical Hodgkin lymphoma bearing the Epstein-Barr virus. |
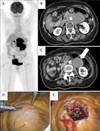 | Fig. 2During evaluation for abdominal pain, a 76-year-old female patient was found to have enlarged paraaortic and mesenteric lymph nodes. (A) Coronal section of fluorine-18 fluorodeoxyglucose positron emission tomography. (B, C) Axial section on computed tomography. The mesenteric lymph nodes were biopsied via the transperitoneal approach (arrow). (D, E) Laparoscopic image. The enlarged lymph node is directly visualized, when the first trocar is placed in a semilateral position. The biopsy was performed by wedge resection. The histopathological diagnosis was adult T-cell leukemia/lymphoma. |
Table 1
The histopathologic diagnosis for all patients
References
1. Silecchia G, Raparelli L, Perrotta N, Fantini A, Fabiano P, Monarca B, et al. Accuracy of laparoscopy in the diagnosis and staging of lymphoproliferative diseases. World J Surg. 2003; 27:653–658.
2. Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematology Am Soc Hematol Educ Program. 2009; 523–531.
3. Bakshi N, Maghfoor I. The current lymphoma classification: new concepts and practical applications triumphs and woes. Ann Saudi Med. 2012; 32:296–305.
4. Asoglu O, Porter L, Donohue JH, Cha SS. Laparoscopy for the definitive diagnosis of intra-abdominal lymphoma. Mayo Clin Proc. 2005; 80:625–631.
5. Bhandarkar DS, Shah RS, Katara AN, Shankar M, Chandiramani VA, Udwadia TE. Laparoscopic biopsy in patients with abdominal lymphadenopathy. J Minim Access Surg. 2007; 3:14–18.
6. Muskat PC, Johnson RA, Bowers GJ. Staging laparotomy in Hodgkin's lymphoma: 1979 to 1988. Am J Surg. 1991; 162:603–606.
7. Vandervelde C, Kamani T, Varghese A, Ramesar K, Grace R, Howlett DC. A study to evaluate the efficacy of image-guided core biopsy in the diagnosis and management of lymphoma--results in 103 biopsies. Eur J Radiol. 2008; 66:107–111.
8. Balestreri L, Morassut S, Bernardi D, Tavio M, Talamini R, Gloghini A, et al. Efficacy of CT-guided percutaneous needle biopsy in the diagnosis of malignant lymphoma at first presentation. Clin Imaging. 2005; 29:123–127.
9. Amador-Ortiz C, Chen L, Hassan A, Frater JL, Burack R, Nguyen TT, et al. Combined core needle biopsy and fine-needle aspiration with ancillary studies correlate highly with traditional techniques in the diagnosis of nodal-based lymphoma. Am J Clin Pathol. 2011; 135:516–524.
10. Daly SC, Klairmont M, Arslan B, Vigneswaran Y, Roggin KF, Ujiki MB, et al. Laparoscopy has a superior diagnostic yield than percutaneous image-guided biopsy for suspected intraabdominal lymphoma. Surg Endosc. 2015; 29:2496–2499.
11. Casaccia M, Torelli P, Cavaliere D, Panaro F, Nardi I, Rossi E, et al. Laparoscopic lymph node biopsy in intra-abdominal lymphoma: high diagnostic accuracy achieved with a minimally invasive procedure. Surg Laparosc Endosc Percutan Tech. 2007; 17:175–178.
12. Gossot D, de Kerviler E, Brice P, Mariette X, Meignin V, Cazals-Hatem D, et al. Surgical endoscopic techniques in the diagnosis and follow-up of patients with lymphoma. Br J Surg. 1998; 85:1107–1110.
13. Mann GB, Conlon KC, LaQuaglia M, Dougherty E, Moskowitz CH, Zelenetz AD. Emerging role of laparoscopy in the diagnosis of lymphoma. J Clin Oncol. 1998; 16:1909–1915.
14. Diulus L, Chalikonda S, Pitt T, Rosenblatt S. Efficacy of laparoscopic mesenteric/retroperitoneal lymph node biopsy. Surg Endosc. 2009; 23:389–393.
15. Hara I, Tanaka K, Yamada Y, Miyake H, Takenaka A, Fujisawa M. Usefulness of laparo- or retroperitoneoscopic biopsy for retroperitoneal lymph node swelling of unknown origin. Int J Urol. 2007; 14:466–469.
16. Clayman RV, Kavoussi LR, Soper NJ, Dierks SM, Meretyk S, Darcy MD, et al. Laparoscopic nephrectomy: initial case report. J Urol. 1991; 146:278–282.
17. Yuan JB, Zu XB, Miao JG, Wang J, Chen MF, Qi L. Laparoscopic pelvic lymph node dissection system based on preoperative primary tumour stage (T stage) by computed tomography in urothelial bladder cancer: results of a single-institution prospective study. BJU Int. 2013; 112:E87–E91.
18. Neyer M, Peschel R, Akkad T, Springer-Stöhr B, Berger A, Bartsch G, et al. Long-term results of laparoscopic retroperitoneal lymph-node dissection for clinical stage I nonseminomatous germ-cell testicular cancer. J Endourol. 2007; 21:180–183.
19. Salky BA, Bauer JJ, Gelernt IM, Kreel I. The use of laparoscopy in retroperitoneal pathology. Gastrointest Endosc. 1988; 34:227–230.
20. Porte H, Copin MC, Eraldi L, Roumilhac D, Jaillard-Thery S, Puech P, et al. Retroperitoneoscopy for the diagnosis of infiltrating retroperitoneal lymphadenopathy and masses. Br J Surg. 1997; 84:1433–1436.
21. Scholz HS, Petru E, Benedicic C, Haas J, Tamussino K, Winter R. Fibrin application for preventing lymphocysts after retroperitoneal lymphadenectomy in patients with gynecologic malignancies. Gynecol Oncol. 2002; 84:43–46.
22. Docimo G, Limongelli P, Conzo G, Gili S, Bosco A, Rizzuto A, et al. Axillary lymphadenectomy for breast cancer in elderly patients and fibrin glue. BMC Surg. 2013; 13:Suppl 2. S8.
23. Benevento R, Santoriello A, Pellino G, Sciaudone G, Candilio G, De Fatico GS, et al. The effects of low-thrombin fibrin sealant on wound serous drainage, seroma formation and length of postoperative stay in patients undergoing axillary node dissection for breast cancer. A randomized controlled trial. Int J Surg. 2014; 12:1210–1215.
24. Navarro-Rodríguez E, Gómez-Luque I, Díaz-Jiménez N, Rioja-Torres P, Bascuñana-Estudillo G, Ruiz-Rabelo JF, et al. Effectiveness of an absorbable fibrin sealant patch to reduce lymphoceles formation after axillary lymphadenectomy for breast cancer: a matched-pair analysis. Am J Surg. 2014; 208:824–830.
25. Marchioni M, Ingrosso M, De Francesco P, Primiceri G, Manco R, Tenaglia RL. The use of haemostatic agents and sealants for the prevention of lymphocele after urological surgery: a review of the literature. Surg Technol Int. 2015; 27:45–50.
26. D'souza MM, Jaimini A, Bansal A, Tripathi M, Sharma R, Mondal A, et al. FDG-PET/CT in lymphoma. Indian J Radiol Imaging. 2013; 23:354–365.
27. Glaudemans AW, de Vries EF, Galli F, Dierckx RA, Slart RH, Signore A. The use of (18)F-FDG-PET/CT for diagnosis and treatment monitoring of inflammatory and infectious diseases. Clin Dev Immunol. 2013; 2013:623036.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download