This article has been corrected. See "Erratum: Correction of the references for Figures. Testosterone therapy in men with testosterone deficiency: Are we beyond the point of no return?" in Volume 58 on page 77.
Abstract
Although testosterone therapy in men with testosterone deficiency was introduced in the early 1940s, utilization of this effective treatment approach in hypogonadal men is met with considerable skepticism and resistance. Indeed, for decades, the fear that testosterone may cause prostate cancer has hampered clinical progress in this field. Nevertheless, even after considerable knowledge was acquired that this fear is unsubstantiated, many in the medical community remain hesitant to utilize this therapeutic approach to treat men with hypogonadism. As the fears concerning prostate cancer have subsided, a new controversy regarding use of testosterone therapy and increase in cardiovascular disease was introduced. Although the new controversy was based on one ill-fated clinical trial, one meta-analysis with studies that utilized unapproved formulation in men with liver cirrhosis, and two retrospective studies with suspect or nonvalidated statistical methodologies and database contaminations, the flames of such controversy were fanned by the lay press and academics alike. In this review we discuss the adverse effect of testosterone deficiency and highlight the numerous proven benefits of testosterone therapy on men's health and debunk the myth that testosterone therapy increases cardiovascular risk. Ultimately, we believe that there is considerable scientific and clinical evidence to suggest that testosterone therapy is safe and effective with restoration of physiological levels in men with testosterone deficiency, irrespective of its etiology.
The last 2 decades have witnessed a revolution in medical management of men with testosterone (T) deficiency (TD; also known as hypogonadism) [123456789101112131415]. TD has been recognized for more than 7 decades [161718] and T therapy has been used clinically, as early as 1940, both in men and women [161718]. However, a lack of understanding of the importance of this hormone in human physiology and in particular men's health became a hurdle in the path of clinical utilization of this therapeutic approach by the medical community to restore normal physiological levels of T in men with TD.
One of the major hurdles concerning T therapy in men with TD is the fear that T causes prostate cancer (PCa) [19]. This premise was based on the observation by Huggins and Hodges in 1941 [20] that T promotes progression of metastatic PCa. While the observation remains accurate, demonstrating that metastatic tumors retain expression and function of the androgen receptors (ARs) and therefore T will promote metastatic tumor growth, no evidence exists to suggest that T per se is a carcinogen that promotes development of PCa [19]. Nevertheless, this dogma was taught to medical students, residents and fellows, for decades, and became a textbook message that T is a carcinogen and causes the development of PCa in men [19]. Although it has never been unequivocally demonstrated that T is an actual carcinogen and indeed promotes development of PCa in men, this myth took hold and attracted followers. However, if this were the case, then one would expect that PCa will be found in all young man over the age 18, since T levels are significantly higher in younger men than in older men [19]. This raises the fundamental question; why PCa is often detected in older men when T levels are significantly low, due to age? This question seems to be either difficult to answer by the myth followers or ignored by the proponents of the theory that "T causes PCa" [21].
A second hurdle hampering the clinical utilization of T therapy in men with TD is the notion that T increases risk of cardiovascular disease (CVD) [22232425]. Such claims were based on flawed studies with no designated primary outcome measures for CVD endpoints [22], retrospective observational studies with suspect methodology and unvalidated statistical methods [2425] or meta-analysis which included data from studies with nonapproved formulations of T in men with severe liver disease [23]. These studies were vastly promoted in clinical circles [26] and the lay media [2728293031323334353637], thus contributing to confusion, hype and hysteria. Furthermore, based on several case reports [38], the U.S. Food and Drug Administration (FDA) added a warning label with regard to the risk of T therapy and venous thromboembolism (VTE). This warning added another road block to advancing T therapy in men with TD. Finally, the FDA also suggested that age-related hypogonadism does not warrant treatment and only TD with recognized etiology merits treatment [39]. In this review, we wish to discuss the adverse impact of TD on men's health and highlight the benefits and risks of T therapy in hypogonadal men. We wish to provoke the readership to critically think about the challenges and opportunities of T therapy and the future of this rapidly changing field in men's health.
TD (hypogonadism) was recognized, as early as 1940s [161718] as significant medical condition, which adversely impacts men's health. TD is associated with increased incidence of metabolic syndrome, obesity, sexual dysfunction, impaired fertility, reduced motivation, increased fatigue, depressed mood, loss of bone and muscle mass, anemia, decreased energy and vigour, insulin resistance, diabetes, inflammation, dyslipidemia, sarcopenia and frailty, reduced quality of life (QoL) and increased mortality [1234567891011121314154041424344]. A substantial body of evidence indicates that coronary artery disease incidence and severity, carotid intima-media thickness, atherosclerosis is inversely correlated with serum T concentrations [45]. There is an urgent need among the medical community for greater awareness of the impact of TD on general health in men with TD.
T elicits a range of physiological processes modulating the physiology and function of multiple organ systems, including muscle, fat, bone, brain, peripheral nerves, and the male genital and reproductive systems. T regulates the metabolism of carbohydrates, lipids and proteins, and influences muscle growth and adipogenesis [56741]. Here in we provide a succinct summary of T therapy benefits on men's health.
Animal studies, with no exception, had demonstrated a critical role for androgens in regulating sexual function [46]. Recent clinical studies [3] have provided considerable evidence that T plays an important role in maintaining sexual function. The association of TD with erectile dysfunction and reduced libido in men is well-established. Observational studies, registries and clinical trials also demonstrated that T therapy produced signif icant improvements in sexual function in men with TD (Table 1) [34748]. Thus the benefit of T therapy on improving sexual function can no longer be ignored or denied. Since sexual health is an integral element of overall health, and sexual dysfunction is associated with depression, reduced mood and loss of self-esteem, it is imperative to state that T therapy is an important therapeutic approach to improve men's health and QoL.
It is universally accepted that T therapy in men with TD increases muscle mass and reduces fat mass (Table 2) [49]. T therapy improves bone mineral density [5051], reduces adipogenesis [5253] and improves anthropometric parameters (Tables 1 and 2) [8]. Most of studies reported to date show that T therapy increases lean body mass (LBM) [4249]. Furthermore, long-term T therapy reduces weight (Fig. 1) [49], waist circumference (Fig. 2) [854], and body mass index (BMI) [84554]. This is not surprising, since T regulates metabolism of carbohydrates, lipids and proteins. Also T regulates muscle growth and function and inhibits adipogenesis. The fact that in some studies the aforementioned benefits were not observed is attributed in part to: (1) inclusion of relatively healthy, nonobese men with normal T levels, (2) short duration of T treatment, (3) varying T formulations used, and (4) poor adherence to T treatment. Nevertheless, the overwhelming consensus is that T therapy improves body composition in men with TD [12].
It may be a surprise to many that T therapy in men with TD was used as early as 1940s to treat angina pectoris and peripheral vascular disease [161718]. The elegant reporting by Lesser [18] in which significant improvement in physical function was demonstrated in subjects with angina pectoris subsequent to T therapy provided a glimpse of the potential role of this powerful hormone in vascular health. The role of androgens in vascular physiology is well-established and it is recognized that T plays an important role in cardiovascular function [567]. A host of cardiovascular risk factors such as obesity, metabolic syndrome, dyslipidemia, hypertension, insulin resistance and diabetes are increased with TD [567]. T therapy improves body composition, reduces body weight, waist circumference, and BMI (Tables 1 and 2) [4841]. T therapy also ameliorates metabolic syndrome components [449], improves lipid profiles (Fig. 3), reduces blood glucose and hemoglobin A1c (Fig. 4), improves insulin sensitivity and reduces inflammation and attenuates systolic and diastolic blood pressure and improves cardio-metabolic functions [128]. These benefits are of significance to attenuation of atherosclerosis and improved cardiovascular health.
The adverse impact of TD on mood and depressive symptoms has been reported in several studies [355565758596061]. Although the magnitude of improvements differed among the various studies, a link between TD, mood and depressive symptoms exists. As pointed out by Khera et al., [58], the association of increased depressive symptoms with TD is confounded by a host of comorbidities that contribute to depression as well as TD. In a multicenter, 12-month observational registry study (n=849) of men with TD treated with 1% T gel, Patient Health Questionnaire (PHQ-9) scores improved significantly (p<0.01) after 3 months of T therapy. By 12-month PHQ-9 scores demonstrated clinically meaningful improvement. In another study in which men (n=886) participating in the Diabetes Prevention Program were treated with metformin, T levels were associated with improvements in mood [59]. In another study Westley et al. [60] showed that depression and/or depressive symptoms were present in 56% of the subjects. In a meta-analysis of 16 randomized trials, Amanatkar et al. [55] found that T therapy had a significant effect on mood in men with TD. Wang et al. [56] demonstrated significant improvement in positive mood and reduction in negative mood with T therapy over a period of 36 months. Observational studies of 799 men on T therapy showed 22% reduction in fatigue scores over 6 months [57]. A modest improvement on global cognition with T therapy was also reported (Table 1) [61].
The fear that T may contribute to PCa and benign prostatic hyperplasia (BPH) had confounded the potential benefits of T therapy in ameliorating lower urinary tract symptoms (LUTS). T protects lower urinary tract (LUT) from metabolic syndrome-induced alterations and T treatment in animals with metabolic syndrome (MetS) counteracts the MetS-induced LUT alterations and attenuates the progression of LUTS [62]. Kathrins et al. [63] found no evidence to support that T therapy worsens LUTS symptoms or increase prostate volume in men with TD. Meuleman et al. [64] also reported that long-term T therapy had no deleterious effects on International Prostate Symptom Score (IPSS) total scores and did not change prostate volume or prostate-specific antigen (PSA) in men with TD. A registry study of 999 men with clinically diagnosed TD showed modest positive effects on LUTS [65]. In a systematic review and meta-analysis, Kohn et al. [66] concluded that T therapy did not worsen LUTS in men with TD. T therapy improved LUTSs in men with TD and mild BPH [6768]. Okada et al. [69] demonstrated that T therapy has considerable therapeutic benefits on LUTS in Japanese men with TD.
Age-related decline in muscle mass and function is known as sarcopenia and is often equated with muscle aging [42]. Clinically, sarcopenia is defined as loss of muscle mass with concomitant deterioration in physical function [4351]. Sarcopenia is attributed, in part, to loss of muscle fiber number and size concomitant with loss of limb motor neurons [70]. Sarcopenia is associated with slow gait speed and low grip strength and is central to development of frailty [42]. Sarcopenia is also associated with limited mobility, higher risk of falls and fractures, impaired physical function, disabilities, loss of independence, institutionalization, hospitalization and increased mortality. Reduced T levels are associated with observed loss of muscle mass and strength [71] and sarcopenia is commonly prevalent in older persons [72].
Considerable evidence exists suggesting that T therapy improves some of the components contributing to frailty and physical decline, such as sarcopenia, muscle strength and physical function [424371]. A number of interventional and observational studies have demonstrated consistently that T therapy improves body composition and contributes to increased LBM and reduced FM [42434450527374757677]. Page et al. [50] demonstrated that T therapy in elderly men (n=70) with mean age of 71 years improved body composition. Srinivas-Shankar et al. [76] reported that in 274 community-dwelling intermediate-frail and frail elderly men at least 65 years of age that T therapy significantly increased LBM and reduced fat mass compared to placebo. These findings are congruent with data reported by Svartberg et al. [52] in which T therapy demonstrated significant improvement in LBM by approximately 4.2 kg and reduced fat mass with concomitant reduction in total body mass. In severely ill patients with liver cirrhosis and sarcopenia, T therapy resulted in an increase in LBM by 4.7 kg after one year of T therapy [78]. Additional evidence is surmised from studies in which T therapy in men with medically induced TD results in a dose-dependent increase in muscle mass and reduction in FM in young and elderly men [79]. Changes in FM correlated inversely with T dose and were significantly different in young vs. older men. Although T therapy consistently increased muscle mass in all reported studies, however, there are inconsistencies with regard to improvement in muscle strength and physical function [80818283].
The relationship between TD and increased all-cause mortality has been reported previously [1] (Table 3). A meta-analysis by Araujo et al. [84] in which 16,184 community dwelling men with mean follow-up of approximately 10 years showed that low T were associated with increased risk of cardiovascular (CV) related mortality. No studies have unequivocally established a direct relationship between low T and mortality, but it has been shown that higher T levels were correlated with reduced mortality and lowest T levels are associated with increased mortality [85]. All the evidence available to date suggest that low T, free T and bioavailable T are associated with increased risk of CV-related and all-cause mortality.
A significant association between TD and mortality was found in most of the studies, irrespective of the type of study. Araujo et al. [84] found that low T levels were associated with an increased risk of CV-related mortality. Yeap et al. [85] also reported higher serum T levels were associated with the lowest mortality and the lowest two quartiles were associated with the higher mortality in men. Yeap et al. [85] reported that higher calculated free T was associated with lower incidence of MI in an unadjusted model. However, this association was lost after adjustment for age and conventional risk factors. Interestingly, however, higher total T or DHT levels were independent and substantive predictors of decreased risk of incident stroke [85].
TD significantly impairs QoL. T therapy improves depression, bone mineral density, energy, libido, erectile function, muscle mass, insulin resistance, and LUTS [86]. Nian et al. [87] reported on the effects of T therapy on QoL and showed that T therapy improves patients' health-related QoL in terms of the decrease in the Aging Male Symptom total score, the psychological, somatic and sexual subscale scores [87]. In a cohort of 223 patients, after adjustments for age and obesity men with normal T levels had significantly better physical and mental health as well as treatment satisfaction responses when compared to those patients with low T levels [88]. It was concluded that men with T levels ≥400 ng/dL reported some improved measures of health-related QoL including greater satisfaction with treatment outcome [88]. Lower T and greater severity of ED independently correlated with poorer physical function, social function, vitality and decline in general health domains of the 36-Item Short Form Survey. This is the first study to report that testosterone deficiency and severity of ED are both independently associated with reduced QoL in men with T2D [8889]. Furthermore, ED and low testosterone are markers of poor health which impact on an individual's self-perception of their health status [89].
The relationship between T and PCa has been a subject of intensive debate [1921]. The notion that T is critical for PCa growth made androgen deprivation therapy (ADT) the mainstay of treatment for advanced PCa. Epidemiological and clinical studies suggested that there is no association between T levels and risk of PCa [6590919293]. There is no evidence to date to suggest that low T levels are protective against PCa and that physiological levels of T increase the risk of PCa. Interestingly, T levels decline with age and PCa incidence increases with age, suggesting that a low T level may contribute to the development of cancer and normal physiological T levels may be protective against PCa [94]. This concept is difficult to grasp in the light of myriad studies linking PCa growth to T on the basis of interpretation of data in which ADT results in regression of metastatic prostate disease. Recently, several studies have demonstrated that ARs in mature prostatic epithelium are critical for maintaining the differentiated phenotype and overall homeostasis of the gland [9095969798]. Haider et al. [93] examined data from 1,023 hypogonadal men receiving testosterone therapy. A total of 11 patients were diagnosed with PCa, with incidence of 54.4 per 10,000 patient-years. Baillargeon et al. [92] also concluded that exposure to T therapy was not associated with an increased risk of high grade PCa or receipt of primary ADT following diagnosis.
Proponents of the T controversy claiming that T therapy increased CVD risk in men with TD have attempted to make a case out of (1) one ill-fated clinical trial [22], which was terminated prematurely in part due to administration of supraphysiological doses of T in elderly frail men with limited mobility, (2) one meta-analysis [23], which included studies that should not have met the criteria for inclusion and (3) two flawed retrospective studies [2425], with unvalidated scientific methods of analyses. These flawed studies were used by the lay media to promote scare tactics, hype and hysteria. A trail of events leading to this hype and hysteria is given in Table 4. It should be noted that some authors have attempted to link T therapy to disease mongering and to inventing a new disease [2699]. These claims are baseless and unfounded in view of the scientific evidence that restoration of physiological levels of T are critical for maintaining men's health. Interestingly, T therapy has been used as early as 1940s with no reported adverse effects on the CV system [121618]. On the contrary, T therapy has been shown to improve the symptoms of angina pectoris and peripheral vascular disease [1618]. A spectrum of recent studies (Table 5) have shown that T therapy does not increase the risk of CVD. In addition, several meta-analyses have shown that T therapy does not increase the risk of CVD (Table 6). The analysis of the FDA [29] in response to a petition to place a black box on T products also concluded that there is no solid evidence that T therapy increases the risk of CVD. The reader is encouraged to read the elegant reviews by Morgentaler et al. [12] and Kloner [100] which addressed this topic in greater depth. Thus, there is no credible evidence to indicate that T therapy increases the risk of CVD. On the contrary, T therapy may be protective [9101112131415].
Several case reports appeared in the literature suggesting that T therapy is associated with serious side effects and increased risk of VTE due to increased hematocrit seen in some men receiving T treatment attributed to testosterone's ability to stimulate erythropoiesis [38]. As a result of these observations the FDA required pharmaceutical companies to add a warning label to their T products to indicate the risk of VTE due to T therapy. The FDA's warning was based entirely on these observational case reports [38]. We should point out that new large observational studies [1115], which investigated the potential adverse effect of T therapy in 30,572 middle-aged in older men and reported that filling a prescription for T therapy was not associated with an increased risk for VTE compared to men without a testosterone prescription [1115]. These findings strongly suggest that T therapy does not increase the risk of VTE in men with TD.
The distortion of the women health initiative (WHI) results both in academic discussions and in the lay media has set fear in the minds of the public, as well as in the medical community. It is disturbing to note that many clinicians will cite the WHI as a reason for not treating men with TD with T therapy. A set of events (Table 4) over the past decade has contributed to the current state of fear, hype and hysteria in the field of T therapy in men with TD. Many of these claims have risen through fear and lack of understanding the physiological role of androgens in human health. In addition, the publication of studies with little or no credible evidence regarding risk of CVD with T therapy [22232425] and the decades long fear of testosterone promoting PCa has compounded this fear [21]. The lay press has also created a negative atmosphere with hype and hysteria around the risks of T therapy that made many clinicians feel that they rather step aside the arena than clearly think about the medical benefits and risks of T therapy [262728293031323334353637]. These factors together with the fear of litigation, at least in the United States, made many in the medical community prefer to shun T therapy than to understand it benefits, risks and health implications. It is not exaggerated to state that the unfamiliarity of many physicians with this complex subspecialty of andrology, which transcends endocrine hormones, cancer, vascular disease and sexual function has also contributed to this unease.
The publication of "Testosterone therapy in older men" [3] should re-ignite the debate on the benefits and harm of T therapy in men. From the outset, we wish to point out that this large study confirms a longstanding and well-documented concept that T therapy has many benefits in men suffering from T deficiency. T therapy is associated with significant improvement in sexual activity, sexual desire and erectile function. Men receiving T therapy reported slightly better mood and lower severity of depressive symptoms. These findings, together with those discussed in this review, show that T therapy has many health related benefits. These include amelioration of the metabolic syndrome components, reduction in fat mass and increased LBM, reduced body weight and waist circumference, increased insulin sensitivity, improved lipid profile, improved blood pressure and reduced inflammatory markers, improved LUTS, reduced mortality and improvement in overall QoL. Thus, the argument that the benefits of T therapy remain unproven should be laid to rest once and for all and health benefits of T therapy can no longer be denied.
The purported adverse ef fects of T therapy on cardiovascular health is based on flawed studies and remains questionable, at best. As pointed out in several observational studies, meta-analyses, and clinical trials [12] T therapy did not cause CVD harm, contrary to previous claims (Tables 5, 6). The findings of a number of studies (Tables 5, 6) dispel claims that men treated with T are at higher risk of myocardial infarctions, stroke and death.
Of importance, the study by Snyder et al. [3] and the resolutions of the consensus panel on T [2] debunked the notion that age-related hypogonadism is not a clinical condition and should remain untreated. As reported in the study [3], T therapy in older men has several benefits and age-related hypogonadism is a clinical condition worthy of treatment. We hope that the findings of this large and well executed study [3] and the summary provided by the consensus panel [2] will serve as a reminder to those who are beating the drums of fear and hysteria on the dangerous use of T in the treatment of men with TD and reassure men suffering from TD and their physicians that such fears and hysteria are unfounded. This study [3] among others should remind all of us to take a step back and bring common sense rather that emotional outbursts to the discussion. This study [3] gives testosterone the chance to be the "Comeback Kid".
Figures and Tables
 | Fig. 1Testosterone therapy in men with testosterone deficiency and differing grade of obesity produces significant and sustained weight loss. Hypogonadal men (n=362) with obesity grade I (Gr. I: n=185; mean age, 58.39±8.04 years), grade II (Gr. II: n=131; mean age, 60.62±5.56 years) and grade III (Gr. III: n=46; mean age, 60.28±5.39 years) treated with testosterone undecanoate injections for up to 6 years. Weight expressed in kilogram. Adapted from Kenny AM, et al. J Am Geriatr Soc 2010;58:1134-43 [74]. |
 | Fig. 2Testosterone therapy in men with testosterone deficiency and differing grade of obesity produces marked and sustained reductions in waist circumference. Waist circumference (WC) (cm) in 362 hypogonadal men with obesity grade I (Gr. I: n=185; mean age, 58.39±8.04 years), grade II (Gr. II: n=131; mean age, 60.62±5.56 years) and grade III (Gr. III: n=46; mean age, 60.28±5.39 years) Treated with testosterone undecanoate injections for up to 6 years. Adapted from Kenny AM, et al. J Am Geriatr Soc 2010;58:1134-43 [74]. |
 | Fig. 3Total cholesterol (A) and low-density lipoprotein (LDL) cholesterol levels (B) and triglyceride levels (C) in men with testosterone (T) deficiency undergoing T therapy for 5 years. Adapted from Traish AM. Am J Physiol Regul Integr Comp Physiol 2016;311:R566-73 [5]. |
 | Fig. 4Glucose concentration (A) and hemoglobin A1c (HbA1c) levels (B) in men with testosterone (T) deficiency undergoing T therapy for 5 years. Adapted from Traish AM. Am J Physiol Regul Integr Comp Physiol 2016;311:R566-73 [5]. |
Table 1
Established benefits of testosterone therapy

Table 2
T Therapy increases lean body mass and reduces total body fat mass in men with
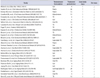
Table 3
Association between low testosterone and all-cause and cardiovascular mortality

Table 4
Events contributing to the testosterone controversy

| 1. | Pearson H. A dangerous elixir? Testosterone therapy jacks up vigour, sex drive and mental acuity — or so proponents claim. But are those who experiment with this potent sex hormone gambling with their health? Nature 2004;431: 500-501. Helen Pearson is a reporter for news@ nature.com and is based in New York. |
| 2. | Basaria S, et al. Adverse events associated with testosterone administration. N Engl J Med 2010;363:109-22. |
| 3. | Gan EH, et al. Many men are receiving unnecessary testosterone prescriptions. BMJ 2012;345:e5469. |
| 4. | Baillargeon J, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med 2013;173:1465-6. |
| 5. | O'connor A. Men’s use of testosterone on the rise. The New York Times. 2013 Jun 3. |
| 6. | Appearance of Low T centers [Internet]. Southlake (TX): Low T Center; [cited 2016 Jul 15]. Available from: https://lowtcenter.com/store_locator/ |
| 7. | Schwartz LM, et al. Low "T" as in "template": how to sell disease. JAMA Intern Med 2013;173:1460-2. |
| 8. | Braun SR. Promoting "low T": a medical writer's perspective. JAMA Intern Med 2013;173:1458-60. |
| 9. | Dubowitz N and Fugh-Berman A. Outside Opinion: Testosterone treatments are dangerous for me. Chicago Tribune. 2013 Sep 15. |
| 10. | Vigen R, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA 2013;310:1829-36. |
| 11. | Baillargeon J, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med 2013;173:1465-6. |
| 12. | Finkle WD, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One 2014;9:e85805. |
| 13. | The Editorial Board. Overselling testosterone, dangerously. New York Times. 2014 Feb 4. |
| 14. | FDA evaluating risk of stroke, heart attack and death with FDA-approved testosterone products [Internet]. Silver Spring (MD): U.S. Food and Drug Administration; 2016 [cited 2016 Jul 15]. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm383904.htm |
| 15. | September 18, 2014: Joint Meeting of the Bone, Reproductive and Urologic Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee Meeting Announcement [Internet]. Silver Spring (MD): U.S. Food and Drug Administration; 2016 [cited 2016 Jul 15]. Available from: http://www.fda.gov/AdvisoryCommittees/Calendar/ucm406131.htm. |
| 16. | Nguyen CP, et al. Testosterone and "Age-Related Hypogonadism"--FDA Concerns. N Engl J Med 2015;373:689-91. |
| 17. | Murphy EN, et al. Doubts about treating hypogonadism due to long-term opioid use with testosterone therapy: a teachable moment. JAMA Intern Med 2014;174:1892-3. |
| 18. | Wierman ME. Risks of different testosterone preparations: too much, too little, just right. JAMA Intern Med 2015;175:1197-8. |
| 19. | Handelsman DJ. Irrational exuberance in testosterone prescribing: when will the bubble burst? Med Care 2015;53:743-5. |
| 20. | Perls T, et al. Disease mongering of age-associated declines in testosterone and growth hormone levels. J Am Geriatr Soc 2015;63:809-11. |
| 21. | Health Canada. Summary safety review - testosterone replacement products – cardiovascular risk [Internet]. Ottawa (ON): Health Canada; 2015 [cited 2016 Jul 15]. Available from: http://www.hc-sc.gc.ca/dhp-mps/medeff/reviews-examens/testosterone-eng.php. |
| 22. | Testosterone products: FDA/CDER statement of risk of venous blood clots [Internet]. Silver Spring (MD): U.S. Food and Drug Administration; 2016 [cited 2015 Jul 26]. Available from: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm402054.htm. |
| 23. | Health Canada. Summary safety review - testosterone replacement products - cardiovascular risk [Internet]. Ottawa (ON): Health Canada; 2015 [cited 2015 Jul 26]. Available from: http://www.hc-sc.gc.ca/dhp-mps/medeff/reviews-examens/testosterone-eng.php. |
| 24. | Layton JB, et al. Comparative safety of testosterone dosage forms. JAMA Intern Med 2015;175:1187-96. |
| 25. | Nguyen CP, et al. Testosterone and "Age-Related Hypogonadism"--FDA Concerns. N Engl J Med 2015;373:689-91. |
Table 5
Testosterone therapy does not increase CV risk

CV, cardiovascular; TT, total testosterone; MI, myocardial infarction; VTE, venous thromboembolism; ICD-9, international classification of diseases, ninth revision; IMS, IMS health is a leading provider of information, services and technology for the healthcare industry around the world; TRT, testosterone replacement therapy; MACE, major adverse cardiovascular.
Table 6
Testosterone therapy does not increase CV risk

References
1. Morgentaler A, Miner MM, Caliber M, Guay AT, Khera M, Traish AM. Testosterone therapy and cardiovascular risk: advances and controversies. Mayo Clin Proc. 2015; 90:224–251.
2. Morgentaler A, Zitzmann M, Traish A, Fox AW, Jones T. Hugh, et al. International expert consensus conference on testosterone deficiency and its treatment- resolutions and conclusions. Mayo Clin Proc. 2016; 91:881–896.
3. Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016; 374:611–624.
4. Traish AM, Haider A, Doros G, Saad F. Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: an observational, long-term registry study. Int J Clin Pract. 2014; 68:314–329.
5. Traish AM. Testosterone therapy in men with testosterone deficiency: are the benefits and cardiovascular risks real or imagined? Am J Physiol Regul Integr Comp Physiol. 2016; 311:R566–R573.
6. Kelly DM, Jones TH. Testosterone: a vascular hormone in health and disease. J Endocrinol. 2013; 217:R47–R71.
7. Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013; 217:R25–R45.
8. Saad F, Yassin A, Doros G, Haider A. Effects of long-term treatment with testosterone on weight and waist size in 411 hypogonadal men with obesity classes I-III: observational data from two registry studies. Int J Obes (Lond). 2016; 40:162–170.
9. Anderson JL, May HT, Lapp in. testosterone replacement therapy on myocardial infarction, stroke, and death in men with low testosterone concentrations in an integrated health care system. Am J Cardiol. 2016; 117:794–799.
10. Sharma R, Oni OA, Gupta K, Chen G, Sharma M, Dawn B, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015; 36:2706–2715.
11. Sharma R, Oni OA, Chen G, Sharma M, Dawn B, Sharma R, et al. Association between testosterone replacement therapy and the incidence of DVT and Pulmonary embolism: a retrospective cohort study of the veterans administration database. Chest. 2016; 150:563–571.
12. Wallis CJ, Lo K, Lee Y, Krakowsky Y, Garbens A, Satkunasivam R, et al. Survival and cardiovascular events in men treated with testosterone replacement therapy: an intention-to-treat observational cohort study. Lancet Diabetes Endocrinol. 2016; 4:498–506.
13. Etminan M, Skeldon SC, Goldenberg SL, Carleton B, Brophy JM. Testosterone therapy and risk of myocardial infarction: a pharmacoepidemiologic study. Pharmacotherapy. 2015; 35:72–78.
14. Baillargeon J, Urban RJ, Kuo YF, Ottenbacher KJ, Raji MA, Du F, et al. Risk of myocardial infarction in older men receiving testosterone therapy. Ann Pharmacother. 2014; 48:1138–1144.
15. Baillargeon J, Urban RJ, Morgentaler A, Glueck CJ, Baillargeon G, Sharma G, et al. Risk of venous thromboembolism in men receiving testosterone therapy. Mayo Clin Proc. 2015; 90:1038–1045.
16. Aub JC, Kety SS. Recent advances in testosterone therapy. N Engl J Med. 1943; 228:338–343.
17. Beaser SB, Massell TB. Therapeutic evaluation of testosterone in peripheral vascular disease. N Engl J Med. 1942; 227:43–44.
18. Lesser MA. Testosterone propionate therapy in one hundred cases of angina pectoris. J Clin Endocrinol Metab. 1946; 6:549–557.
19. Morgentaler A. Testosterone therapy can be given to men with no concern that it will promote prostate cancer development or progression: pro. J Urol. 2016; 196:985–988.
20. Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972; 22:232–240.
21. Gleave ME, Klotz L. Testosterone therapy can be given to men with no concern that it will promote prostate cancer development or progression: con. J Urol. 2016; 196:985–988.
22. Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010; 363:109–122.
23. Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013; 11:108.
24. Vigen R, O'Donnell CI, Barón AE, Grunwald GK, Maddox TM, Bradley SM, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013; 310:1829–1836.
25. Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, Cook MB, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014; 9:e85805.
26. Perls T, Handelsman DJ. Disease mongering of age-associated declines in testosterone and growth hormone levels. J Am Geriatr Soc. 2015; 63:809–811.
27. The Editorial Board. Overselling testosterone, dangerously [internet]. The New York Times;2014. 02. 04. cited 2014 Feb 5. Opinion page. Available from: http://www.nytimes.com/2014/02/05/opinion/overselling-testosterone-dangerously.html.
28. Wolfe S, Carome M. Petition to the FDA on all testosterone products [Internet]. Public Citizen;2014. cited 2014 Feb 25. Available from: http://www.citizen.org/documents/2184.pdf.
29. Woodcock. Citizen petition denial response from FDA CDER to public citizen [Internet]. Silber Spring (MD): U.S. Food and Drug Administration;2014. cited 2014 Aug 31. Available from: http://www.citizen.org/documents/2020_FDA%20Final%20Response%20to%20Petition.pdf.
30. Quick study: testosterone supplement may have cardiovascular risks for older men [Internet]. The Washington Post;2010. 07. 13. cited 2016 Jul 15. Available from: http://www.washingtonpost.com/wp-dyn/content/article/2010/07/12/AR2010071204179.html.
31. Testosterone treatments linked to heart risks [Internet]. USA TODAY;2013. 11. 05. cited 2014 Jan 29. Available from: http://www.usatoday.com/story/news/nation/2013/11/05/testosterone-heart-attacks/3448543/.
32. Singer N. Selling that new-man feeling [Internet]. The New York Times;2013. 11. 23. cited 2016 Jul 15. Available from: http://www.nytimes.com/2013/11/24/business/selling-that-new-man-feeling.html.
33. Silverman E. Doctors try to influence a medical journal poll on testosterone treatments [Internet]. The Wall Street Journal;2014. 12. 02. cited 2014 Dec 2. Available from: http://blogs.wsj.com/pharmalot/2014/12/02/doctors-try-to-influence-a-medical-journal-poll-on-testerone-treatments/.
34. Beck M. In men's fight against aging, how much risk to take? The FDA is weighing whether testosterone-replacement therapy is safe. [Internet]. The Wall Street Journal;2014. 10. 27. cited 2014 Oct 27. Available from: http://www.wsj.com/articles/in-mens-fight-against-aging-how-much-risk-to-take-1414443065.
35. Sifferlin A. Low-T drugs and heart risk: here's the latest [Internet]. Time;2015. 08. 11. cited 2015 Aug 11. Available from: http://time.com/3992444/testosterone-heart-disease/.
36. Lester M. Are you man enough? the truth about low testosterone. Time;2014. 07. 31. cited 2016 Jul 15. Available from: http://time.com/3063213/low-testosterone/.
37. Endocrine Society. Too many men take testosterone when they don't need it [Internet]. The Washington Post;2014. 01. 13. cited 2014 Jan 13. Available from: https://www.washingtonpost.com/national/health-science/too-many-men-take-testosterone-when-they-dont-need-it/2014/01/13/d2ecc700-7956-11e3-af7f-13bf0e9965f6_story.html.
38. Glueck CJ, Wang P. Testosterone therapy, thrombosis, thrombophilia, cardiovascular events. Metabolism. 2014; 63:989–994.
39. Nguyen CP, Hirsch MS, Moeny D, Kaul S, Mohamoud M, Joffe HV. Testosterone and "Age-Related Hypogonadism"--FDA Concerns. N Engl J Med. 2015; 373:689–691.
40. Antonio L, Wu FC, O'Neill TW, Pye SR, Carter EL, Finn JD, et al. Associations between sex steroids and the development of metabolic syndrome: a longitudinal study in European men. J Clin Endocrinol Metab. 2015; 100:1396–1404.
41. Traish AM, Zitzmann M. The complex and multifactorial relationship between testosterone deficiency (TD), obesity and vascular disease. Rev Endocr Metab Disord. 2015; 16:249–268.
42. O'Connell MD, Tajar A, Roberts SA, Wu FC. Do androgens play any role in the physical frailty of ageing men? Int J Androl. 2011; 34:195–211.
43. Albert SG, Morley JE. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: a systematic review. Clin Endocrinol (Oxf). 2016; 85:436–443.
44. Corona G, Giagulli VA, Maseroli E, Vignozzi L, Aversa A, Zitzmann M, et al. Testosterone supplementation and body composition: results from a meta-analysis of observational studies. J Endocrinol Invest. 2016; 39:967–981.
45. Aversa A, Bruzziches R, Francomano D, Rosano G, Isidori AM, Lenzi A, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebocontrolled study. J Sex Med. 2010; 7:3495–3503.
46. Traish AM, Park K, Dhir V, Kim NN, Moreland RB, Goldstein I. Effects of castration and androgen replacement on erectile function in a rabbit model. Endocrinology. 1999; 140:1861–1868.
47. Brock G, Heiselman D, Maggi M, Kim SW, Rodr SW, Rodr SW, et al. Effect of testosterone solution 2% on testosterone concentration, sex drive and energy in hypogonadal men: results of a placebo controlled study. J Urol. 2016; 195:699–705.
48. Finkelstein JS, Lee H, Burnett-Bowie SA, Pallais JC, Yu EW, Borges LF, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013; 369:1011–1022.
49. Traish AM. Testosterone and weight loss: the evidence. Curr Opin Endocrinol Diabetes Obes. 2014; 21:313–322.
50. Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, Bremner WJ, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005; 90:1502–1510.
51. Morley JE. Pharmacologic options for the treatment of sarcopenia. Calcif Tissue Int. 2016; 98:319–333.
52. Svartberg J, Agledahl I, Figenschau Y, Sildnes T, Waterloo K, Jorde R. Testosterone treatment in elderly men with subnormal testosterone levels improves body composition and BMD in the hip. Int J Impot Res. 2008; 20:378–387.
53. Singh R, Artaza JN, Taylor WE, Braga M, Yuan X, Gonzalez-Cadavid NF, et al. Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with beta-catenin and T-cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology. 2006; 147:141–154.
54. Francomano D, Bruzziches R, Barbaro G, Lenzi A, Aversa A. Effects of testosterone undecanoate replacement and withdrawal on cardio-metabolic, hormonal and body composition outcomes in severely obese hypogonadal men: a pilot study. J Endocrinol Invest. 2014; 37:401–411.
55. Amanatkar HR, Chibnall JT, Seo BW, Manepalli JN, Grossberg GT. Impact of exogenous testosterone on mood: a systematic review and meta-analysis of randomized placebo-controlled trials. Ann Clin Psychiatry. 2014; 26:19–32.
56. Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, et al. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 2004; 89:2085–2098.
57. Pexman-Fieth C, Behre HM, Morales A, Kan-Dobrosky N, Miller MG. A 6-month observational study of energy, sexual desire, and body proportions in hypogonadal men treated with a testosterone 1% gel. Aging Male. 2014; 17:1–11.
58. Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. The effect of testosterone supplementation on depression symptoms in hypogonadal men from the Testim Registry in the US (TRiUS). Aging Male. 2012; 15:14–21.
59. Kim C, Barrett-Connor E, Aroda VR, Mather KJ, Christophi CA, Horton ES, et al. Testosterone and depressive symptoms among men in the Diabetes Prevention Program. Psychoneuroendocrinology. 2016; 72:63–71.
60. Westley CJ, Amdur RL, Irwig MS. High rates of depression and depressive symptoms among men referred for borderline testosterone levels. J Sex Med. 2015; 12:1753–1760.
61. Wahjoepramono EJ, Asih PR, Aniwiyanti V, Taddei K, Dhaliwal SS, Fuller SJ, et al. The effects of testosterone supplementation on cognitive functioning in older men. CNS Neurol Disord Drug Targets. 2016; 15:337–343.
62. Vignozzi L, Morelli A, Sarchielli E, Comeglio P, Filippi S, Cellai I, et al. Testosterone protects from metabolic syndrome-associated prostate inflammation: an experimental study in rabbit. J Endocrinol. 2012; 212:71–84.
63. Kathrins M, Doersch K, Nimeh T, Canto A, Niederberger C, Seftel A. The relationship between testosterone-replacement therapy and lower urinary tract symptoms: a systematic review. Urology. 2016; 88:22–32.
64. Meuleman EJ, Legros JJ, Bouloux PM, Johnson-Levonas AO, Kaspers MJ, Elbers JM, et al. Effects of long-term oral testosterone undecanoate therapy on urinary symptoms: data from a 1-year, placebo-controlled, dose-ranging trial in aging men with symptomatic hypogonadism. Aging Male. 2015; 18:157–163.
65. Debruyne FM, Behre HM, Roehrborn CG, Maggi M, Wu FC, Schr Mag FH, et al. Testosterone treatment is not associated with increased risk of prostate cancer or worsening of lower urinary tract symptoms: prostate health outcomes in the Registry of Hypogonadism in Men. BJU Int. 2016; 07. 13. [Epub]. DOI: 10.1111/bju.13578.
66. Kohn TP, Mata DA, Ramasamy R, Lipshultz LI. Effects of testosterone replacement therapy on lower urinary tract symptoms: a systematic review and meta-analysis. Eur Urol. 2016; 69:1083–1090.
67. Shigehara K, Sugimoto K, Konaka H, Iijima M, Fukushima M, Maeda Y, et al. Androgen replacement therapy contributes to improving lower urinary tract symptoms in patients with hypogonadism and benign prostate hypertrophy: a randomised controlled study. Aging Male. 2011; 14:53–58.
68. Ko YH, Moon du G, Moon KH. Testosterone replacement alone for testosterone deficiency syndrome improves moderate lower urinary tract symptoms: one year follow-up. World J Mens Health. 2013; 31:47–52.
69. Okada K, Miyake H, Ishida T, Sumii K, Enatsu N, Chiba K, et al. Improved lower urinary tract symptoms associated with testosterone replacement therapy in japanese men with lateonset hypogonadism. Am J Mens Health. 2016; 06. 02. [Epub]. DOI: 10.1177/1557988316652843.
70. Tomlinson BE, Irving D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci. 1977; 34:213–219.
71. Bhasin S. Testosterone supplementation for aging-associated sarcopenia. J Gerontol A Biol Sci Med Sci. 2003; 58:1002–1008.
72. von Haehling S, Morley JE, Anker SD. From muscle wasting to sarcopenia and myopenia: update 2012. J Cachexia Sarcopenia Muscle. 2012; 3:213–217.
73. Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Holmes JH, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab. 1999; 84:1966–1972.
74. Kenny AM, Kleppinger A, Annis K, Rathier M, Browner B, Judge JO, et al. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J Am Geriatr Soc. 2010; 58:1134–1143.
75. Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, et al. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002; 282:E601–E607.
76. Srinivas-Shankar U, Roberts SA, Connolly MJ, O'Connell MD, Adams JE, Oldham JA, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010; 95:639–650.
77. Caminiti G, Volterrani M, Iellamo F, Marazzi G, Massaro R, Miceli M, et al. Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure a double-blind, placebo-controlled, randomized study. J Am Coll Cardiol. 2009; 54:919–927.
78. Sinclair M, Grossmann M, Hoermann R, Angus PW, Gow PJ. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: a randomised controlled trial. J Hepatol. 2016; 65:906–913. DOI: 10.1016/j.jhep.2016.06.007.
79. Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005; 90:678–688.
80. Eichholzer M, Barbir A, Basaria S, Dobs AS, Feinleib M, Guallar E, et al. Serum sex steroid hormones and frailty in older American men of the Third National Health and Nutrition Examination Survey (NHANES III). Aging Male. 2012; 15:208–215.
81. Mohr BA, Bhasin S, Kupelian V, Araujo AB, O'Donnell AB, McKinlay JB. Testosterone, sex hormone-binding globulin, and frailty in older men. J Am Geriatr Soc. 2007; 55:548–555.
82. Hyde Z, Flicker L, Almeida OP, Hankey GJ, McCaul KA, Chubb SA, et al. Low free testosterone predicts frailty in older men: the health in men study. J Clin Endocrinol Metab. 2010; 95:3165–3172.
83. Tajar A, O'Connell MD, Mitnitski AB, O'Neill TW, Searle SD, Huhtaniemi IT, et al. Frailty in relation to variations in hormone levels of the hypothalamic-pituitary-testicular axis in older men: results from the European male aging study. J Am Geriatr Soc. 2011; 59:814–821.
84. Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011; 96:3007–3019.
85. Yeap BB, Alfonso H, Chubb SA, Handelsman DJ, Hankey GJ, Almeida OP, et al. In older men an optimal plasma testosterone is associated with reduced all-cause mortality and higher dihydrotestosterone with reduced ischemic heart disease mortality, while estradiol levels do not predict mortality. J Clin Endocrinol Metab. 2014; 99:E9–E18.
86. Khera M. Male hormones and men's quality of life. Curr Opin Urol. 2016; 26:152–157.
87. Nian Y, Ding M, Hu S, He H, Cheng S, Yi L, et al. Testosterone replacement therapy improves health-related quality of life for patients with late-onset hypogonadism: a meta-analysis of randomized controlled trials. Andrologia. 2016; 07. 08. [Epub]. DOI: 10.1111/and.12630/abstract;jsessionid=A784F440904F991C3EFA21E5E0DDD560.f02t02.
88. Cohen A, Lapin B, Wang CH, Helfand B, Victorson D, Novakovic K. Variation in testosterone levels and health-related quality of life in men diagnosed with prostate cancer on active surveillance. Urology. 2016; 94:180–187.
89. Brooke JC, Walter DJ, Kapoor D, Marsh H, Muraleedharan V, Jones TH. Testosterone deficiency and severity of erectile dysfunction are independently associated with reduced quality of life in men with type 2 diabetes. Andrology. 2014; 2:205–211.
90. Simanainen U, Allan CM, Lim P, McPherson S, Jimenez M, Zajac JD, et al. Disruption of prostate epithelial androgen receptor impedes prostate lobe-specific growth and function. Endocrinology. 2007; 148:2264–2272.
91. Endogenous Hormones and Prostate Cancer Collaborative Group. Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008; 100:170–183.
92. Baillargeon J, Kuo YF, Fang X, Shahinian VB. Long-term exposure to testosterone therapy and the risk of high grade prostate cancer. J Urol. 2015; 194:1612–1616.
93. Haider A, Zitzmann M, Doros G, Isbarn H, Hammerer P, Yassin A. Incidence of prostate cancer in hypogonadal men receiving testosterone therapy: observations from 5-year median followup of 3 registries. J Urol. 2015; 193:80–86.
94. Prehn RT. On the prevention and therapy of prostate cancer by androgen administration. Cancer Res. 1999; 59:4161–4164.
95. Wu CT, Altuwaijri S, Ricke WA, Huang SP, Yeh S, Zhang C, et al. Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc Natl Acad Sci U S A. 2007; 104:12679–12684.
96. Niu Y, Altuwaijri S, Lai KP, Wu CT, Ricke WA, Messing EM, et al. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proc Natl Acad Sci U S A. 2008; 105:12182–12187.
97. Bonaccorsi L, Muratori M, Carloni V, Marchiani S, Formigli L, Forti G, et al. The androgen receptor associates with the epidermal growth factor receptor in androgen-sensitive prostate cancer cells. Steroids. 2004; 69:549–552.
98. Leotoing L, Manin M, MontM D, Baron S, Communal Y, Lours C, et al. Crosstalk between androgen receptor and epidermal growth factor receptor-signalling pathways: a molecular switch for epithelial cell differentiation. J Mol Endocrinol. 2007; 39:151–162.
99. Fugh-Berman A. Should family physicians screen for testosterone deficiency in men? No: screening may be harmful, and benefits are unproven. Am Fam Physician. 2015; 91:226–228.
100. Kloner RA. Testosterone replacement therapy: new data on efficacy and cardiovascular safety. J Cardiovasc Pharmacol Ther. 2016; 04. 28. [Epub]. DOI: 10.1177/1074248416646938.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download