Abstract
Materials and Methods
The records of 2,339 patients whose UC was diagnosed between January 1974 and December 2012 were reviewed. All data about characteristics of patients, of UC and, of SPC was, recorded digitally. We investigated the prevalence and the type of second or higher order cancers, and the factors associated with SPC.
Results
Total 260 patients (11.1%) had SPC, 14 had a third primary cancer and one had a fourth primary cancer. The most common SPC with UC was lung cancer (29.6%). Of all 260 with SPC, 64 (24.6%) had synchronous (within the 6 months) SPC, 120 (46.2%) had subsequent SPC and, 76 (29.2%) had antecedent SPC. The mean duration of SPC was 56 months in patients with subsequent SPC and 75.8 months in patients with antecedent SPC. The mean age at the time of diagnosis of UC was higher in patients with SPC. The ratio of male gender, body mass index, blood type, status of smoking and, occupational risk was similar in both groups. Total amount of smoking and the mean follow-up were higher in patients with SPC.
In 1889, Billroth et al. [1] first reported multiple primary cancers (MPCs) in a single patient. The most accepted definition of MPCs is that given by Warren and Gates [2], who state that each neoplasm must represent a distinct malignancy and that a metastatic origin must be excluded.
The higher cancer risk in older patients is a reflection of the incidence of MPCs increasing with age. The incidence of genitourinary cancer in patients with MPCs is reported as 13.5% [3]. The most common malignancy pairing is prostate cancer and urothelial cancer (UC) in patients with MPCs (12%).
Patients with nonmuscle invasive UC have a long survival time and a long follow-up period [4]. Patients with nonmetastatic muscle invasive UC have a long survival time after successful treatment [5]. The incidence of UC and MPCs is increasing with age. During the long follow-up period for patients with UC, physicians who follow them have chance to prevent the patients from other possible diseases. We aimed to underline the potential risk of having second malignancies and potential chance to detect them earlier. To clarify this, we investigated MPCs in patients with UC in a retrospective cohort study.
This study began after the local ethics committee gave approval. The study data was derived from the onco-urology clinic at Tepecik Research and Education Hospital in İzmir, Turkey. All patients with urinary cancer were followed at this clinic. The records of 2,339 patients' whose UC were diagnosed between January 1974 and December 2012 were reviewed. All data about the characteristics of patients (age, sex, occupation, smoking history, coexisting disease, detailed medical history, date of death or last visit, cause of death), of UC (date of diagnosis, type of diagnosis, histology, stage, size, location, initial treatment, date of all recurrences, date of progression), and of MPCs (date of diagnosis, type of diagnosis, histology) were recorded digitally.
MPCs were diagnosed according to Warren and Gates' criteria [2]. MPCs diagnosed within 6 months were considered synchronous; otherwise, they were considered metachronous. The patients with and without MPCs were compared. All factors were investigated for any association with MPC. All data were transferred to a computer environment and statistical analysis was performed by software (SPSS ver. 17.0, SPSS Inc., Chicago, IL, USA). The results were expressed as mean±standard deviation. Categorical and continuous variables were analyzed with chi-square and Student t-tests respectively. Multivariate logistic regression analysis was done for all factors. A p-value of <0.05 was considered statistically significant.
Of the 2,339 patients, 88.5% were male. The median age at the time of diagnosis was 63 years (range, 16–113 years). Of the patients, 260 (11.1%) had a second primary cancer (SPC). Of those 260, 14 had a third primary cancer and one had a fourth primary cancer. The most common SPC with UC was lung cancer (29.6%). Prostate cancer, gastrointestinal system tumors, and larynx cancer were the next most common SPCs (Table 1). The percentages of the most common SPCs for all 2,224 male patients are shown in Table 2. The mean duration between UC and SPC was 56.5 months (median, 32 months). Of all 260 patients with an SPC, 64 (24.6%) had synchronous SPC, 120 (46.2%) had subsequent SPC, and 76 (29.2%) had antecedent SPC. The mean duration of SPC was 56 months in patients with subsequent SPC and 75.8 months in patients with antecedent SPC. Male patients were more likely to have subsequent SPC, while the opposite was true for female patients (Table 3).
The patients were divided into two groups—UC without SPC (group 1, n=2,079) and UC with SPC (group 2, n=260). The mean age at the time of UC diagnosis was higher in group 2. The ratio of male gender, body mass index, blood type, smoking status, and occupational risk was similar in both groups. The total amount of smoking and the mean follow-up were higher in patients with SPC (Table 4). Tumor characteristics were compared, and the stage, grade, size, number, and type of tumor were not significantly different between the groups. However, the CIS ratio was similar (Table 5).
Long follow-ups for oncologic patients as a result of increased life expectancy and improved cancer therapy was quarreled us a new situation of multiple cancers. Patients with cancer have an increased risk of having other neoplasms due to factors such as (1) exposure to common carcinogens, (2) genetic predisposition, (3) frequent examination for follow-up and, (4) previous chemo or radiotherapy history. In combination with these factors, increased survival rates allow for a longer time for the development of new cancers.
An analysis of the National Cancer Institute's Surveillance, Epidemiology and End Results (NCI-SEER) database has revealed that approximately 16% of new cancers are second or higher order cancers [6]. In other words, 13.7% of cancer survivors developed second or higher order cancer during 25 years of follow-up [7]. In a large series of 4,209 patients with primary urologic malignancy (1,899 patients with UC), Wegner [8] reported 144 patients (3.3%) with MPC. In our study, which has the largest series in the literature (2,339 patients with UC), we found MPC in 11.1% of patients with a primary urologic malignancy. We found 14 cases of triple neoplasms (5.4% of all SPCs) and 1 case of 4 neoplasms. Wegner [8] reported 18 cases of triple neoplasms (12.5% of all SPCs).
Powell et al. [9] reported prostate cancer as the most common first cancer type. Lung cancers were the most common second neoplasms. In their urologic carcinoma series, Wegner [8] reported that prostate cancer and bladder cancer were the most common pairs. In another study of all cancer types in our country, bladder cancer was the second most common primary cancer and lung cancer was the most common SPC [10]. Coyte et al. [11] demonstrated that patients with bladder cancers were at increased risk of lung cancer and prostate cancer. We found that lung cancer is the most common cancer paired with UC and prostate cancer is the second most common. We considered the fact that lung cancer has similar predisposing factors, such as smoking, and that it is the most common cancer in men in Izmir [12]. The incidence of prostate cancer detection while treating patients for bladder cancer is increasing. This is because there is no screening protocol for prostate cancer in our country.
According to the national cancer registry [12], the prevalence of lung cancer among males in our province is 85 per 100,000. In our study, lung cancer occurred in 3.4% of male patients with UC. This means lung cancer is 40 times more common in males with UC. According to the same registry, the prevalence of prostate cancer was 38 per 100,000. We detected a 2.7% incidence of prostate cancer in men with UC, which is 70 times more than among the general population. Physicians following patients with UC should look for SPCs during follow-up.
We found 24.6% synchronous SPCs, whereas Powell et al. [9] reported 24.5% and Wegner [8] reported 43%. Powell et al. [9] reported that lung cancer and bladder cancer (tobacco-associated) are more likely to be synchronous primary cancers. In our study, we found that lung cancer was commonly a subsequent metachronous cancer, and larynx cancer (also tobacco-associated) was more likely to be an antecedent metachronous cancer. Wegner [8] found 30-month intervals between first and second cancers in men and 110-month intervals for women. They also found that for men half of the cancers were synchronous. We found only a quarter of the cancers to be synchronous and found shorter intervals between first and second cancers. We also found that SPCs in men were more likely to be subsequent cancers and SPCs in women were more likely to be antecedent.
As a result of long follow-up periods, the chances of detecting SPCs are improving. We found a longer follow-up period for patients with SPCs. Wegner [8] reported that the mean age when a first primary cancer is detected is 66.6 and the mean age when an SPC is detected is 70.5. Within the four years after the first primary cancer, SPC was detected. The incidence of MPCs increases with age [13]. We also found that patients with SPC were older compared to those without SPC.
The association between the increased risk of various cancers and tobacco exposure is well known [14]. Gursel et al. [10] states that half of patients with MPC are smokers. This ratio increases to 66% for males. In our UC cohort, we had a male dominancy and nearly 80% of our patients with SPC were smokers, 60% of which were nonstop smokers. However, there was no difference compared to patients without SPC. Otunctemur et al. [15] investigated 25 patients with UC and smoking-related SPC. They studied whether the cancer of patients with smoking-related SPC was more aggressive. Contrarily, we detected similar tumor characteristics for patients with or without SPC. In terms of the factors investigated, only the total amount of smoking (daily cigarettes×years) was associated with SPC in our study. A particular factor predicting the risk of SPC could not be identified in this study. Further studies will address this issue.
Patients with cancer are more inclined to have another cancer. Increased survival and longer follow-up periods facilitate the detection of second or higher order malignancies. The majority of patients with UC have long life expectancy. In patients with UC, the risk of having another cancer is significantly higher than for the general population. Physicians managing patients with UC should look for SPCs.
Figures and Tables
Table 1
Distribution of multiple primary cancer
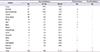
Table 2
Distribution of second primary cancer in men

| Cancer | Second primary, n (%) |
|---|---|
| Lung | 76 (3.4) |
| Prostate | 61 (2.7) |
| Gastrointestinal | 39 (1.8) |
| Larynx | 19 (0.9) |
Table 3
Distribution of second primary cancer according to appearance

Table 4
Comparison of groups due to demographics

Table 5
Comparison of groups due to tumor characteristics

References
1. Billroth T. General surgical pathology and therapy. Guidance for students and physicians. Lecture. Khirurgiia (Mosk). 1991; (10):136–143.
2. Warren S, Gates O. Multiple primary malignant tumors: a survey of the literature and a statistical study. Am J Cancer. 1932; 16:1358–1414.
3. Ray P, Sharifi R, Ortolano V, Guinan P. Involvement of the genitourinary system in multiple primary malignant neoplasms: a review. J Clin Oncol. 1983; 1:574–581.
4. Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Compérat E, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013; 64:639–653.
5. Witjes JA, Compérat E, Cowan NC, De Santis M, Gakis G, Lebret T, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol. 2014; 65:778–792.
6. Travis LB. The epidemiology of second primary cancers. Cancer Epidemiol Biomarkers Prev. 2006; 15:2020–2026.
7. SEER Cancer statistics Review, 1975-2003 [Internet]. Bethesda (MD): National Cancer Institute;2006. cited 2012 Aug 27. Available from: http://seer.cancer.gov/archive/csr/1975_2003/http://seer.cancer.gov/archive/csr/1975_2003/.
8. Wegner HE. Multiple primary cancers in urologic patients. Audit of 19-year experience in Berlin and review of the literature. Urology. 1992; 39:231–236.
9. Powell S, Tarchand G, Rector T, Klein M. Synchronous and metachronous malignancies: analysis of the Minneapolis Veterans Affairs (VA) tumor registry. Cancer Causes Control. 2013; 24:1565–1573.
10. Gursel B, Meydan D, Özbek N, Ozdemir O, Odabas E. Multiple primary malignant neoplasms from the black sea region of Turkey. J Int Med Res. 2011; 39:667–674.
11. Coyte A, Morrison DS, McLoone P. Second primary cancer risk - the impact of applying different definitions of multiple primaries: results from a retrospective population-based cancer registry study. BMC Cancer. 2014; 14:272.
12. Eser S, Yakut C, Ozdemir R, Karakilinç H, Ozalan S, Marshall SF, et al. Cancer incidence rates in Turkey in 2006: a detailed registry based estimation. Asian Pac J Cancer Prev. 2010; 11:1731–1739.
13. Pastore AL, Palleschi G, Autieri D, Leto A, Ripoli A, Maggioni C, et al. Synchronous primary neoplasms of the bladder, skin and breast in a male patient: a case report. World J Surg Oncol. 2013; 11:282.
14. Wynder EL, Mushinski MH, Spivak JC. Tobacco and alcohol consumption in relation to the development of multiple primary cancers. Cancer. 1977; 40:4 Suppl. 1872–1878.
15. Otunctemur A, Koklu I, Ozbek E, Dursun M, Sahin S, Besiroglu H, et al. Are bladder neoplasms more aggresive in patients with a smoking-related second malignancy? Asian Pac J Cancer Prev. 2014; 15:4025–4028.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download