Abstract
Purpose
Based on the experiences of our center, we sought to verify the necessity of actively removing stones during retrograde intrarenal surgery (RIRS) for the management of renal stones.
Materials and Methods
From March 2010 to March 2015, 248 patients underwent RIRS at our center. We classified these patients into 2 groups according to the performance of active stone removal; group A (n=172) included the patients whose stones were actively removed using a stone basket, and group B (n=76) included the patients whose stones were fragmented with laser lithotripsy without active removal of the fragments. We retrospectively compared the operation time, success rate, and complication rate between the 2 groups.
Results
There were no significant differences between groups A and B in terms of mean age (56.1 years vs. 58.6 years), male to female ratio (115:57 vs. 46:30), mean body mass index (24.5 kg/m2 vs. 25.0 kg/m2), mean preoperative size of stone (11.1 mm vs. 11.1 mm), the ratio of unilateral and bilateral stones (136:36 vs. 64:12), success rate (89.0% vs. 86.8%), operation time (82.5 minutes vs. 82.1 minutes), overall complication rate (9.9% vs. 11.8%), incidence of febrile urinary tract infection (6.4% vs. 2.6%), gross hematuria (1.7% vs. 2.6%), or postoperative de novo hydronephrosis (2.9% vs. 5.3%).
The global prevalence of renal stone disease was reported at 3%–15%, and its prevalence is increasing [1]. Traditionally, renal stones have been treated with open surgery; currently, a few minimally invasive treatment modalities such as extracorporeal shock wave lithotripsy (ESWL), percutaneous nephrolithotomy (PCNL), retrograde intrarenal surgery (RIRS), and laparoscopic surgery can be used in place of open surgery. The choice of procedure among these treatment modalities mainly depends on the size and location of the stones, the patients' characteristics, and the preferences of the surgeon [2345]. Recently, with the development of flexible ureteroscopy and the holmium laser, RIRS has been gaining popularity for use in the management of small to intermediate-sized renal stones, and its effectiveness and safety have been proven by many studies [567].
Since the first flexible ureteroscopic procedures were introduced in the 1960s, flexible ureteroscopy has evolved in design and application. As improvements have been made to the flexible ureteroscope, such as multiple working channels, a smaller caliber, greater resolution, and extended field of vision, RIRS has become more widely performed. Highly qualified imaging apparatuses, along with the versatile mobile capabilities of flexible ureteroscopes, enable easy access into the calyceal systems. Moreover, progress in surgical skill has increased stone-free rates. Given the advances in equipment and techniques, RIRS has widened its indications and is now considered the primary treatment modality for the management of small to intermediate-sized renal stones.
Typically, most RIRS procedures have consisted of ureteral access sheath insertion, flexible ureteroscope engagement, identification of stones, laser lithotripsy, removal of stone fragments, and double J stent insertion. The process of stone extraction using a stone basket has been considered an important step in increasing stone-free rates. However, it may also increase operation time and the risk of complications, such as ureteral injury, in the event of large stones. Hence, another strategy called 'dusting' has been introduced, whereby the stones are transformed into very tiny fragments without being actively removed to induce the natural drainage of stone fragments postoperatively.
However, a limited number of studies to date have compared this technique, i.e., fragmentation only without active stone removal, with the traditional technique, which involves fragmentation plus the active removal of stones, in terms of success rate, operation time, and complication rate. Therefore, we compared surgical outcomes between these two techniques based on experiences at our center and sought to verify the necessity of the active removal of stones during RIRS.
From March 2010 to March 2015, 248 consecutive patients underwent RIRS at Kyungpook National University Hospital, and a single surgeon, who had experienced over 100 cases of RIRS, performed all operations using an 8.2-F flexible ureteroscope (URF P-5, Olympus, Tokyo, Japan) with a 200-µ holmium laser (Lumenis, Tel Aviv, Israel). The Institutional Review Board of the Kyungpook National University Hospital (approval number: 2016-04-021) approved the study protocol based on the Declaration of Helsinki. Patients' preoperative clinical data including age, gender, body mass index (BMI), and stone characteristics such as number, size, multiplicity, laterality, and location were retrospectively reviewed through medical records. Before the operation, all patients were evaluated by physical examination, routine blood tests, urinalysis, urine culture, and radiologic images, including simple X-ray, renal ultrasound (US), and noncontrasted computed tomography (CT). Prophylactic antibiotics were routinely used in all patients on the day of operation. Patients whose urine cultures demonstrated bacterial growth on preoperative evaluation were treated with appropriate antibiotics, and the operation was performed after sterile urine was confirmed.
The operation was performed under general or spinal anesthesia in the lithotomy position in all patients. After cystoscopy, a hydrophilic guidewire was inserted into the ureter. A semirigid ureteroscope was introduced to visualize the ureter and facilitate placement. A ureteral access sheath (12/14 F) was placed in all cases, after which a flexible ureteroscope was introduced. Lithotripsy was performed with a laser lithotripter. Irrigation during RIRS was provided manually by an assistant. In the active stone removal group, a 1.9-F nitinol stone basket (Zero-tip, Boston Scientific, Malborough, MA, USA) was used after the fragmentation procedure to remove fragments from the collecting system. At the end of the procedure, fluoroscopy was performed to evaluate stone clearance, and a 6-F double-J stent was routinely placed and maintained for 1 or 2 weeks in all patients.
We classified all patients into 2 groups according to the performance of active stone removal; group A (n=172) included the patients whose stones were actively removed using a stone basket (Fig. 1), and group B (n=76) included the patients whose stones were merely fragmented with laser lithotripsy without being actively removed (Fig. 2). We performed the active stone removal technique for the first 172 consecutive patients, and the other 76 consecutive patients underwent dusting, the fragmentation-only technique. We compared operation time, success rate, and complications between the 2 groups, retrospectively. Stone size was determined by measuring the longest axis on preoperative CT scan. Surgical success was defined as completely stone-free or with clinically insignificant remnant stones (<3 mm) on CT scan one month after the operation without any related symptoms.
For statistical evaluations, IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA) was used, and p<0.05 (two-tailed) was considered statistically significant. Chi-square test was used to determine difference in proportions for categorical data such as gender, laterality of stone, stone free status, and complications, while continuous variables such as age, BMI, stone size, and operation time were assessed using Student t-tests.
The mean age, male to female ratio, and mean BMI of groups A and B were 56.1 years vs. 58.6 years (p=0.137), 115:57 vs. 46:30 (p=0.206), and 24.5 kg/m2 vs. 25.0 kg/m2 (p=0.393), respectively. The preoperative mean stone size, ratio of unilateral and bilateral stones, Hounsfield unit (HU), ratio of upper, mid, and lower calyx stones in groups A and B were 11.1 mm vs. 11.1 mm (p=0.946), 136:36 vs. 64:12 (p=0.222), 905.0 vs. 860.8 (p=0.457), and 29:49:64 vs. 13:22:41 (p=0.497), respectively (Table 1). There were no significant differences in success rate (89.0% vs. 86.8%) and operation time (82.5 minutes vs. 82.1 minutes) between the 2 groups. Likewise, the overall rates of complication (9.9% vs. 11.8%), incidence of febrile urinary tract infection (UTI) (6.4% vs. 2.6%), gross hematuria (1.7% vs. 2.6%), and de novo hydronephrosis after the operation (2.9% vs. 5.3%) were not significantly different between the 2 groups (Table 2). In our study, the complications encountered were all Clavien-Dindo grade 1 (n=15) or 2 (n=12) according to modified Clavien-Dindo classification, and severe complications of grade 3 or greater were not observed.
The first reported flexible ureteroscopic procedure was performed by Marshal in 1964 in order to observe a distal ureteric stone. Since then, the surgical instruments, imaging apparatuses, and level of surgical skill have progressed greatly, and RIRS is now widely performed [567]. The 2015 European Association of Urology Guidelines on Urolithiasis stated that RIRS is recommended as a valid treatment alternative to ESWL for stones less than 1 cm in size in the renal pelvis and upper and middle calyces [8]. RIRS is even considered the primary treatment of choice for small to intermediate-sized lower calyceal stones [9].
RIRS has several advantages compared to ESWL. RIRS allows direct stone fragmentation under endoscopic direct vision and the fragmented stones can then be removed actively using a stone basket. This procedure results in superior stone-free rates, especially for lower calyceal stones. Cecen et al. [10] reported that RIRS and ESWL have similar stone-free (92% vs. 87%, p=0.270) and retreatment (7.5%) rates for the treatment of upper or mid calyx stones of 10–20 mm. They also reported that there was no statistical difference in complication rates between RIRS and ESWL (22.8% vs. 16.7%). However, the use of RIRS for lower calyceal stones is preferable to ESWL, because it can incorporate the stone extraction procedure. Recently, El-Nahas et al. [11] performed a comparative study of RIRS and ESWL in patients with lower pole stones of 10–20 mm. In this study, they reported that the stone-free rates, complication rates, and mean operative time of RIRS and ESWL were 86.5% and 67.7% (p=0.038), 13.5% and 4.8% (p=0.146), and 73 minutes and 92 minutes (p=0.018), respectively.
During RIRS, the procedure of active stone extraction using a stone basket is considered a critical step toward increasing the stone-free rate. However, it may also increase operation time and the risk of complications such as ureteral injury, especially if frequent stone retrieval is performed without the use of a ureteral access sheath. Furthermore, it can cause severe complications such as stone or stone basket entrapment in the ureter if the stone size is not small enough to pass through the ureter. Thus, another strategy called 'dusting' has been recently introduced, whereby the stones are fragmented into very tiny pieces without being actively removed, to induce the natural drainage of stone fragments postoperatively. Although no study has specifically compared the use of active stone removal and dusting techniques during RIRS, various related studies have been reported. Regardless of whether or not the stones are actively removed, overall stone-free rates range from 85% to 98.7% as reported in the literature [12131415]. The overall success rates of RIRS in our study were similar to these previous studies. To increase stone-free rate in fragmentation-only group, we provided a continuous high-pressure irrigation flow to flush out the stone fragments, especially for lower calyceal stones, via manual irrigation after completing lithotripsy. In addition, patients were instructed to hydrate excessively and to change body position frequently for at least 1 to 2 weeks postoperatively. Although RIRS using the fragmentation-only technique in our study did not achieve a higher success rate than expected, it also showed comparable results compared to those of other studies.
The mean operation times of the 2 groups were also not statistically different in our study. Before the analysis, we assumed that the mean operation time of group B (fragmentation-only group) might be shorter than group A (active stone removal group). The difference in lasing time between the 2 groups can be presumed to be a contributing factor. The stones are usually fragmented into much smaller pieces when performing the fragmentation-only technique compared to the standard technique, because tinier fragments may be more easily passed postoperatively. In contrast, it is not necessary to make the fragments very small when performing the active stone removal technique. In fact, if the stone fragments are too small, the process of collecting the fragments with the stone basket becomes more difficult. Therefore, although stone extraction time was not a factor in group B, we assumed that the lasing time would be longer in this study. However, since this study was retrospectively performed and the exact lasing time was not recorded in the medical records, this assumption could not be confirmed.
The operation time in our study seemed longer compared with the results of recent studies that reported mean operation times at 35–70 minutes [1416171819]. We presumed that the relatively longer operation time in our study might be due to differences in both the definition of operation time and the surgical steps compared with other studies. In our study, operation time was defined as the time of anesthesia induction to the end of anesthesia, while other studies defined operation time as the time of insertion of the cystoscope to the completion of stent insertion. Furthermore, in our study, we routinely introduced the semirigid ureteroscope in all patients during the ureteral examination. Therefore, this also could be one of the reasons for the prolonged operation times.
RIRS has been reported to have a 9%–25% complication rate [14171820]. In previous studies, infection (febrile UTI) was the most common complication following RIRS, while ureteral injury was the most serious. In most of the cases in our study, the complications encountered were minor, of Clavien-Dindo grade 1 or 2 only, which was similar to previously reported studies. The overall complication rates of the 2 groups in our study were 11.0% and 10.5%, respectively, and febrile UTI was the most common complication. There were no significant differences in complication type or rate between the active stone removal and fragmentation-only groups in our study. Overall, it seems that the fragmentation-only technique is also comparable to the standard technique in RIRS in terms of safety.
To the best of our knowledge, this is the first study to compare the efficacy and safety of the active stone removal and fragmentation-only techniques in RIRS, but there are some limitations to this study. First, as a retrospective study, we did not perform a randomized case-controlled study with a detailed analysis of operation time; for example, semirigid ureteroscopic working time, flexible ureteroscopic working time, lasing time, stone extraction time, and stent insertion time were not separately measured. Second, a comparison of the stone analysis results in the 2 groups was not performed due to the lack of stone analysis results in the fragmentation-only group. Instead, we indirectly compared preoperative stone characteristics using HU measured by preoperative CT. Although it does not reflect exact stone characteristics, the hardness of the stones can be predicted by measuring HU. Thus, a prospective randomized controlled trial that includes a more detailed analysis of operation time and stone characteristics will be necessary in the future.
This study demonstrated that the fragmentation-only technique, without removal of the stone fragments, showed comparable results in terms of success rate, operation time, and complication rate compared to the technique involving the active removal of stone fragments during RIRS. Although a further randomized prospective study is needed, this technique appears to be a safe and effective option in RIRS.
Figures and Tables
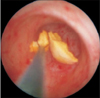 | Fig. 1An endoscopic image of active removal of stone using stone basket during retrograde intrarenal surgery. |
Table 1
Comparison of preoperative patient characteristics between groups A and B

Table 2
Comparison of perioperative surgical outcomes between groups A and B

References
1. Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. 2010; 12:e86–e96.
2. Srisubat A, Potisat S, Lojanapiwat B, Setthawong V, Laopaiboon M. Extracorporeal shock wave lithotripsy (ESWL) versus percutaneous nephrolithotomy (PCNL) or retrograde intrarenal surgery (RIRS) for kidney stones. Cochrane Database Syst Rev. 2009; (4):CD007044.
3. Argyropoulos AN, Tolley DA. Evaluation of outcome following lithotripsy. Curr Opin Urol. 2010; 20:154–158.
4. Scales CD Jr, Krupski TL, Curtis LH, Matlaga B, Lotan Y, Pearle MS, et al. Practice variation in the surgical management of urinary lithiasis. J Urol. 2011; 186:146–150.
5. Bader MJ, Eisner B, Porpiglia F, Preminger GM, Tiselius HG. Contemporary management of ureteral stones. Eur Urol. 2012; 61:764–772.
6. Breda A, Ogunyemi O, Leppert JT, Schulam PG. Flexible ureteroscopy and laser lithotripsy for multiple unilateral intrarenal stones. Eur Urol. 2009; 55:1190–1196.
7. Riley JM, Stearman L, Troxel S. Retrograde ureteroscopy for renal stones larger than 2.5 cm. J Endourol. 2009; 23:1395–1398.
8. Turk C, Petrik A, Sarica K, Seitz C, Skolarikos A, Straub M, et al. EAU Guidelines on diagnosis and conservative management of urolithiasis. Eur Urol. 2016; 69:468–474.
9. Bas O, Bakirtas H, Sener NC, Ozturk U, Tuygun C, Goktug HN, et al. Comparison of shock wave lithotripsy, flexible ureterorenoscopy and percutaneous nephrolithotripsy on moderate size renal pelvis stones. Urolithiasis. 2014; 42:115–120.
10. Cecen K, Karadag MA, Demir A, Bagcioglu M, Kocaaslan R, Sofikerim M. Flexible Ureterorenoscopy versus Extracorporeal Shock Wave Lithotripsy for the treatment of upper/middle calyx kidney stones of 10-20 mm: a retrospective analysis of 174 patients. Springerplus. 2014; 3:557.
11. El-Nahas AR, Ibrahim HM, Youssef RF, Sheir KZ. Flexible ureterorenoscopy versus extracorporeal shock wave lithotripsy for treatment of lower pole stones of 10-20 mm. BJU Int. 2012; 110:898–902.
12. Huang Z, Fu F, Zhong Z, Zhang L, Xu R, Zhao X. Flexible ureteroscopy and laser lithotripsy for bilateral multiple intrarenal stones: is this a valuable choice? Urology. 2012; 80:800–804.
13. Resorlu B, Issi Y, Onem K, Germiyanoglu C. Management of lower pole renal stones: the devil is in the details. Ann Transl Med. 2016; 4:98.
14. Bozkurt OF, Resorlu B, Yildiz Y, Can CE, Unsal A. Retrograde intrarenal surgery versus percutaneous nephrolithotomy in the management of lower-pole renal stones with a diameter of 15 to 20 mm. J Endourol. 2011; 25:1131–1135.
15. Kruck S, Anastasiadis AG, Herrmann TR, Walcher U, Abdelhafez MF, Nicklas AP, et al. Minimally invasive percutaneous nephrolithotomy: an alternative to retrograde intrarenal surgery and shockwave lithotripsy. World J Urol. 2013; 31:1555–1561.
16. Sabnis RB, Jagtap J, Mishra S, Desai M. Treating renal calculi 1-2 cm in diameter with minipercutaneous or retrograde intrarenal surgery: a prospective comparative study. BJU Int. 2012; 110(8 Pt B):E346–E349.
17. Akman T, Binbay M, Ozgor F, Ugurlu M, Tekinarslan E, Kezer C, et al. Comparison of percutaneous nephrolithotomy and retrograde flexible nephrolithotripsy for the management of 2-4 cm stones: a matched-pair analysis. BJU Int. 2012; 109:1384–1389.
18. Bryniarski P, Paradysz A, Zyczkowski M, Kupilas A, Nowakowski K, Bogacki R. A randomized controlled study to analyze the safety and efficacy of percutaneous nephrolithotripsy and retrograde intrarenal surgery in the management of renal stones more than 2 cm in diameter. J Endourol. 2012; 26:52–57.
19. Pan J, Chen Q, Xue W, Chen Y, Xia L, Chen H, et al. RIRS versus mPCNL for single renal stone of 2-3 cm: clinical outcome and cost-effective analysis in Chinese medical setting. Urolithiasis. 2013; 41:73–78.
20. Ozturk U, Sener NC, Goktug HN, Nalbant I, Gucuk A, Imamoglu MA. Comparison of percutaneous nephrolithotomy, shock wave lithotripsy, and retrograde intrarenal surgery for lower pole renal calculi 10-20 mm. Urol Int. 2013; 91:345–349.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download