Abstract
Purpose
Materials and Methods
Results
Figures and Tables
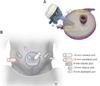 | Fig. 1(A) GelSeal cap prepared with the 12-mm camera port and 10-mm sleeve port. (B) Port placement for robot-assisted partial cystectomy. |
 | Fig. 3(A, B) Ultrasound confirmation of tumor location using the drop-down robotic ultrasound probe. |
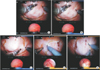 | Fig. 4(A-E) Progressive circumferential scoring of the peritoneal aspect of the bladder around the tumor with a 10- to 20-mm margin. |
 | Fig. 5(A) Extracting the partial cystectomy specimen for bimanual and frozen-section assessment. (B, C) Bimanual examination and frozen-section analysis. |
Table 1
Demographics and preoperative data on patients undergoing robotic partial cystectomy

SD, standard deviation; BMI, body mass index; UC, urothelial carcinoma; ASA, American Society of Anesthesiologists.
a:Abdominal surgery included appendectomy, open inguinal hernia repair, and cesarean section in the 3 patients with history of abdominal surgery; b:Risk factors included smoking in 3 patients and Cyclophosphamide in 1 patient (treated for B-cell skin lymphoma [case 6]); c:Symptoms included hematuria or lower urinary tract symptoms; d:High=grade 3, intermediate=grade 2, low=grade 1 [World Health Organization 1973]; e:The site involved in the single patient labeled as 'other' was posterior upper part of the bladder, left of the midline [case 7]; f:In the patient [case 6] in whom multifocality was noted - all three tumor masses were clustered together on the dome without any additional satellite lesions; g:Prior therapy for bladder cancer included either previous Bacillus Calmette-Guerin and/or transurethral resection of the bladder tumor with curative intent.
Table 2
Intraoperative, perioperative and follow-up outcomes in patients undergoing robotic partial cystectomy

NA, not applicable; RAPC, robot-assisted partial cystectomy; USG, ultrasonogram; SD, standard deviation; UC, urothelial carcinoma; Nx, not assessed; POD, postoperative day.
a:There were no differences in the operative and console times among RAPC (first 2 cases) and m-RAPC (last 5 cases) with p-value being 0.67 and 0.81, respectively; b:Not applicable to the initial 2 patients [i.e., cases 1 and 2]; c:For final pathology only 6 cases were evaluable; One case reported as 'NA' had no assessable tumor in the partial cystectomy specimen (pT0 [case 2]); d:High=grade 3, intermediate=grade 2, low=grade 1 [World Health Organization 1973]; e:Composite surgical margin status refers to the sum total of the result of the surgical margin of the excised partial cystectomy specimen and the additional tissue excised, wherever applicable; f:One patient developed lymphocele requiring drain placement (Clavien-Dindo grade IIIa); g:Minimum follow-up of 12 months with a median follow-up of 38.9 months (interquartile range, 15.9–53.3 months); Based on surveillance cystoscopy and urine cytology (One patient [case 6] developed a superficial recurrence [Ta disease] 6 months postoperatively and was treated with TURBT and was tumor free at 9 months but had recurrence again at his latest follow-up at 12 months); h:The survey was administered at each patient's latest follow-up; i:All patients answered "none of the time" to all the 3 regret questions; j:One patient (case 6) passed away a week after his latest follow-up at 12 months because of reasons unrelated to bladder cancer—died of Lewy-body disease.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download