Abstract
Purpose
To investigate whether relaxation of the rat penile corpus cavernosum could be controlled with NOBL-1, a novel, light-controllable nitric oxide (NO) releaser.
Materials and Methods
Fifteen-week-old male Wistar-ST rats were used. The penile corpus cavernosum was prepared and used in an isometric tension study. After noradrenaline (10-5 M) achieved precontraction, the penile corpus cavernosum was irradiated by light (470–500 nm) with and without NOBL-1 (10-6 M). In addition, we noted rats' responses to light with vardenafil (10-6 M), a phosphodiesterase-5 (PDE-5) inhibitor. Next, responses to light in the presence of a guanylate cyclase inhibitor, ODQ (1H-[1,2,4] oxadiazolo[4,3-a]quinoxalin-1-one) (10-5 M), were measured. All measurements were performed in pretreated L-NAME (10-4 M) conditions to inhibit endogenous NO production.
Results
Corpus cavernosal smooth muscle, precontracted with noradrenaline, was unchanged by light irradiation in the absence of NOBL-1. However, in the presence of NOBL-1, corpus cavernosal smooth muscle, precontracted with noradrenaline, relaxed in response to light irradiation. After blue light irradiation ceased, tension returned. In addition, the light response was obviously enhanced in the presence of a PDE-5 inhibitor.
Conclusions
This study showed that rat corpus cavernosal smooth muscle relaxation can be light-controlled using NOBL-1, a novel, light sensitive NO releaser. Though further in vivo studies are needed to investigate possible usefulness, NOBL-1 may be prove to be a useful tool for erectile dysfunction therapy, specifically in the field of penile rehabilitation.
Nitric oxide (NO) is a key mediator for penile erection [12]. In the penis, NO is normally produced by neuronal and endothelial NO synthase (nNOS and eNOS). The nNOS-derived NO is involved in initiation of penile erection, while eNOS-derived NO is involved in maintaining the penile erection [3]. The endogenous NO moves into corpus cavernosum smooth muscle cells, activates guanylate cyclase (GC), and then converts guanosine 5'-triphosphate to cyclic guanosine monophosphate (cGMP). Next, cGMP activates cGMP-specific protein kinase and causes relaxation of the corpus cavernosum smooth muscle, which allows penile erection [4]. Phosphodiesterase-5 (PDE-5) degrades cGMP following erection initiation [56].
As a first therapy for erectile dysfunction (ED), PDE-5 inhibitors are used to increase cGMP concentration; however, in some patients with diabetes-induced ED, those who are postprostatectomy, or have other types of severe vascular dysfunction, a PDE-5 inhibitor may not necessarily improve erectile function [7]. Saenz de Tejada et al. [8] reported that patients with ED and diabetes had an impaired endothelialderived NO-stimulated relaxation response in the penile corpus cavernosum. In these cases, increasing NO production may be key to improving erectile function, while PDE-5 inhibitor effects may be low. Making this NO-based therapy potentially even more attractive to clinicians and patients, there are currently no noninvasive and effective therapies for penile rehabilitation. Thus, controlling NO production may be a novel, condition-specific, noninvasive therapy for ED and/or penile rehabilitation. Accordingly, we created a tool to facilitate this task, the NO releaser, NOBL-1.
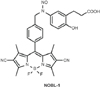
Designed by Ieda et al. [9], NOBL-1 is a light-controllable releaser of NO (Fig. 1). Though some light-sensitive NO donors already exist, they are limited by factors such as cytotoxicity, due to transition metal components, or expense, requiring two photon devices [910]. NOBL-1 does not contain transition metals that can cause cytotoxicity and is composed of N-nitrosoaniline and a BODIPY dye. In spite of the carcinogenic property of the N-nitroso structure, Namiki et al. [11] reported that the N-nitrosoaniline structure did not show strong toxicity, and BODIPY dyes have been utilized for molecular imaging owing to their less cytotoxic fluorescent molecules [12]. In addition, NOBL-1's NO production can be easily controlled by blue light [9]. Considering these advantages, we aimed to determine whether NOBL-1 could be an effective and simple ED therapy by allowing light-controlled relaxation of the penile corpus cavernosal smooth muscle.
Fifteen-week-old male Wistar-ST rats (SLC, Shizuoka, Japan) were used in this study. Animals were housed in a temperature and humidity-controlled room with a 12-hour light/dark cycle and free access to laboratory chow and water. All experiment protocols were performed with approval by the Animal Care and Use Committee of Nagoya City University.
Penises were obtained from rats after euthanasia. The tunica albuginea, urethra, and veins were removed. The remaining corpus cavernosum was prepared in a chilled Krebs solution composed of 119 mM NaCl, 4.6 mM KCl, 1.5 mM CaCl2, 1.2 mM MgCl2, 15 mM NaHCO3, 1.2 mM NaH2PO4, and 11 mM glucose. One side of the prepared corpus cavernosum was clipped, and the other side was ligated and connected to a force transducer (ADInstruments, Bella Vista, Australia). The force transducer was connected to a bridge amp and PowerLab 4/26 (ADInstruments), and tension was measured using LabChart 7 software (ADInstruments). The experimental tools were placed in a dark room to avoid ambient room light (Fig. 2A).
Measurements were taken at least 1 hour af ter collecting and preparing the corpus cavernosum. The corpus cavernosum was treated with L-NAME (10-4 M) to inhibit endogenous NO production.
First, to measure the effects of light irradiation in the absence of NOBL-1, noradrenaline (10-5 M) was allowed to promote precontraction, and the sample was irradiated with light for 15 and 30 seconds (wavelength 470–500 nm, intensity 31 mW/cm2). Light was irradiated from outside of the organ bath by MAX-302 (Asahi spectra, Tokyo, Japan) in the dark room (Fig. 2B, C). Next, NOBL-1 (10-6 M) was added, and the sample received light for a further 15 and 30 seconds.
Next, to investigate the synergistic effects of PDE-5 inhibitors, the sample was washed in Kreb's solution, vardenafil (10-6 M) was added, and the corpus cavernosum was precontracted by noradrenaline (10-5 M). Then, NOBL-1 (10-6 M) was added to the organ bath, and the sample received light for 15 and 30 s.
To confirm that these effects were indeed induced via the NO-GC-cGMP pathway, we then added a GC inhibitor, (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one, ODQ) (10-5 M), and measured light-induced relaxation in the presence of NOBL-1.
In the absence of NOBL-1, light irradiation did not cause any change in the corpus cavernosum (Fig. 3, left); however, when the corpus cavernosum smooth muscle was precontracted by noradrenaline and NOBL-1 was added, the sample relaxed when irradiated with light. The relaxation responses were maintained during 15- and 30-second intervals (Fig. 3, right), although the tension returned when the light was removed (Fig. 3, right).
Upon examining the synergistic effects between NOBL-1 and vardenafil, a PDE-5 inhibitor, we saw that the corpus cavernosum's light-induced relaxation response was obviously enhanced as a result of vardenafil pretreatment (Fig. 4). In this scenario, tension was maintained both during and after irradiation (Fig. 4).
The results of analysis showed Fig. 5. The penile corpus cavernosum were significantly relaxed with the response to light both with only NOBL-1 and NOBL-1 under vardenafil compared with the response to light without NOBL-1.
To confirm whether those responses were induced by NO, we examined the sample's response to light in the presence of a GC inhibitor, ODQ. The response to light irradiation completely disappeared in the presence of ODQ treatment, suggesting that the relaxation response occurred via the GC pathway (Fig. 6).
This study showed that the corpus cavernosum's relaxation response was controllable by NOBL-1 and light irradiation. In addition, the response was enhanced in the presence of vardenafil. When treated with ODQ, the relaxation response ceased, suggesting that NO, known to be both the cause of this relaxation response and ultimately initiate and maintain penile erection, was produced by the effects of NOBL-1 and light irradiation. In turn, this suggests that NOBL-1 might be useful for treating ED caused by low levels of NO. This phenomenon may also be useful for more general penile rehabilitation.
Penile rehabilitation may be performed for patients who develop ED after undergoing radical prostatectomy [13]. Penile rehabilitation commonly focuses on three interrelated concepts as follows: (1) improving cavernosal oxygenation, (2) preserving endothelial structure and function, and (3) preventing smooth muscle structural changes [14]. Currently, injecting prostaglandin E1 into the corpus cavernosum or long-term treatment with PDE-5 inhibitors are the standard therapies for these patients [1314151617]. Unfortunately, prostaglandin E1 injections are invasive and may cause pain, and PDE-5 inhibitor regimens may not be useful if low levels of NO are the cause of the ED. nNOS plays an essential role in initiating erection by NO production from the nitrergic nerve [3]. Bilateral transection of cavernous nerves was reported to cause a reduction in the nNOS-positive nerve fibers from 3 weeks after surgery, and regeneration was not observed in an animal study [18]. Thus, the reduction in nNOS is suggested to be a cause of ED after radical prostatectomy. In these cases, NOBL-1 and light irradiation may be a more effective rehabilitation method.
Patients with ED have generally been treated with oral PDE-5 inhibitors as a first-line therapy, although approximately 30% of patients fail to respond to this treatment. Common causes of this response failure are diabetes mellitus and severe neurological or vascular disease [7192021]. Only if NO is being produced after sexual arousal can PDE-5 inhibitors affect penile tissue; in the aforementioned conditions, NO production may be too low to initiate PDE-5 effects or entirely nonexistent. On the other hand, in this study, NOBL-1 could freely initiate and maintain an erection in the presence of light without any need for sexual stimulation. We also investigated whether NOBL-1 and light irradiation could still produce a relaxation response in the presence of a PDE-5 inhibitor, since patients may still take PDE-5 inhibitors. As expected, PDE-5 inhibitors enhanced the relaxation response. Thus, a combination of NOBL-1 and a PDE-5 inhibitor treatment might be effective for ED. In that case, attention to hypotension is considered necessary, although the method that uses NOBL-1 and light irradiation can produce NO focally.
In addition to its occasional lack of effectiveness, some patients treated with PDE-5 inhibitor therapy also experience systemic side effects such as headaches, flushing, dyspepsia, and back pain [19]. Because NOBL-1 was able to produce site-specific NO upon light irradiation, such adverse events may be less likely to occur using this novel therapy.
This study did have some limitations. Primarily, the use of rat models makes generalizability to humans difficult, and the small sample size and short follow-up should be addressed in future efforts to improve novel methods of penile rehabilitation.
In conclusion, the corpus cavernosum's relaxation response was controlled by light irradiation in the presence of NOBL-1. In addition, pretreatment with vardenafil enhanced the relaxation response. Both with and without pretreatment, tension returned after irradiation ceased. Therefore, NOBL-1 might be a useful therapy for ED and penile rehabilitation.
Figures and Tables
Fig. 2
Evaluation system. (A) This work was performed in a dark room. White arrows represent the light source. (B) Penile tissue in light-irradiated organ bath. (C) Pattern diagram of evaluation system. Light was irradiated from outside the organ bath.

Fig. 3
Corpus cavernosum's response to light irradiation without (left) and with NOBL-1 (right). The yellow line represents light irradiation: short width, 15 seconds; long width, 30 seconds. NA, noradrenaline (10-5 M); NOBL-1 (10-6 M).

Fig. 4
Corpus cavernosum's response to light irradiation with NOBL-1 and vardenafil. The yellow line represents light irradiation: short width, 15 seconds; long width, 30 seconds. Var, vardenafil (10-6 M); NA, noradrenaline (10-5 M); NOBL-1 (10-6 M).

References
1. Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH. Nitric oxide: a physiologic mediator of penile erection. Science. 1992; 257:401–403.
2. Ignarro LJ, Bush PA, Buga GM, Wood KS, Fukuto JM, Rajfer J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem Biophys Res Commun. 1990; 170:843–850.
3. Hurt KJ, Musicki B, Palese MA, Crone JK, Becker RE, Moriarity JL, et al. Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proc Natl Acad Sci U S A. 2002; 99:4061–4066.
4. Hosogai N, Takakura S, Manda T, Mutoh S. Enzyme activities of the nitric oxide-cGMP pathway in corpus cavernosum isolated from middle-aged rats. Eur J Pharmacol. 2003; 473:65–70.
5. Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995; 75:725–748.
6. Soderling SH, Beavo JA. Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr Opin Cell Biol. 2000; 12:174–179.
7. McMahon CG. Erectile dysfunction. Intern Med J. 2014; 44:18–26.
8. Saenz de Tejada I, Goldstein I, Azadzoi K, Krane RJ, Cohen RA. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989; 320:1025–1030.
9. Ieda N, Hotta Y, Miyata N, Kimura K, Nakagawa H. Photomanipulation of vasodilation with a blue-light-controllable nitric oxide releaser. J Am Chem Soc. 2014; 136:7085–7091.
10. Nakagawa H, Hishikawa K, Eto K, Ieda N, Namikawa T, Kamada K, et al. Fine spatiotemporal control of nitric oxide release by infrared pulse-laser irradiation of a photolabile donor. ACS Chem Biol. 2013; 8:2493–2500.
11. Namiki S, Kaneda F, Ikegami M, Arai T, Fujimori K, Asada S, et al. Bis-N-nitroso-caged nitric oxides: photochemistry and biological performance test by rat aorta vasorelaxation. Bioorg Med Chem. 1999; 7:1695–1702.
12. Ueno T, Urano Y, Kojima H, Nagano T. Mechanism-based molecular design of highly selective fluorescence probes for nitrative stress. J Am Chem Soc. 2006; 128:10640–10641.
13. Montorsi F, Guazzoni G, Strambi LF, Da Pozzo LF, Nava L, Barbieri L, et al. Recovery of spontaneous erectile function after nerve-sparing radical retropubic prostatectomy with and without early intracavernous injections of alprostadil: results of a prospective, randomized trial. J Urol. 1997; 158:1408–1410.
14. Kim JH, Lee SW. Current status of penile rehabilitation after radical prostatectomy. Korean J Urol. 2015; 56:99–108.
15. Mulhall JP, Bella AJ, Briganti A, McCullough A, Brock G. Erectile function rehabilitation in the radical prostatectomy patient. J Sex Med. 2010; 7(4 Pt 2):1687–1698.
16. Chung E, Brock G. Sexual rehabilitation and cancer survivorship: a state of art review of current literature and management strategies in male sexual dysfunction among prostate cancer survivors. J Sex Med. 2013; 10:Suppl 1. 102–111.
17. Salonia A, Burnett AL, Graefen M, Hatzimouratidis K, Montorsi F, Mulhall JP, et al. Prevention and management of postprostatectomy sexual dysfunctions part 2: recovery and preservation of erectile function, sexual desire, and orgasmic function. Eur Urol. 2012; 62:273–286.
18. Carrier S, Zvara P, Nunes L, Kour NW, Rehman J, Lue TF. Regeneration of nitric oxide synthase-containing nerves after cavernous nerve neurotomy in the rat. J Urol. 1995; 153:1722–1727.
19. Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013; 381:153–165.
20. Heidelbaugh JJ. Management of erectile dysfunction. Am Fam Physician. 2010; 81:305–312.
21. Smith WB 2nd, McCaslin IR, Gokce A, Mandava SH, Trost L, Hellstrom WJ. PDE5 inhibitors: considerations for preference and long-term adherence. Int J Clin Pract. 2013; 67:768–780.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download