Abstract
Recent advances in cancer nanomedicine have attracted remarkable attention in medical sectors. Pharmacologic research on nanomedicines, including targeted cancer therapy, has increased dramatically in the past 5 years. The success stories of nanomedicines in the clinical field include the fabrication of nanomedicines that show maximum loading efficiency into carriers, maximal release kinetics, and minimum toxicity to healthy cells. Nanoparticle-mediated medicines have been developed to specifically target prostate cancer tissue by use of aptamers, antibody targeting, and sustained release of nanomedicines in a dose- and time-dependent manner. Nanomedicines have been developed for therapeutic application in combination with image-guided therapy in real time. The scope of one of these nanomedicines, Abraxane (paclitaxel), may be extended to prostate cancer therapeutic applications for better quality of patient life and longer survival. This review provides an update on the latest directions and developments in nanomedicines for prostate cancer.
Prostate cancer (PCa) has mostly affected the male population of the United States, and in 2012 US cases constituted approximately 29% (240,890) of the incidence of PCa [1]. In South Korea, the incidence of PCa has significantly increased while the mortality rate has steadily decreased. From 2007 to 2013, the incidence PCa in Korean men doubled (5,516 to 10,855 per year), while the prevalence tripled (18,830 to 51,411) during the same period. The mortality rate increased slightly, from 4.2 in 2000 to 5.9 in 2007 and 6.0 in 2013 (per 100,000, age-adjusted). PCa incidence increased significantly faster in men <70 years of age than in the older age group [2].
The emergence of nanotechnology has drawn much attention in the 21st century for applications in medicine such as "nanomedicine." Nanomedicine combines engineering, physics, biology, chemistry, mathematics, and medicine and strives to improve disease detection, imaging, and drug delivery through the use of nanodevices [3]. Nanotechnology encompasses the materials, devices, and delivery systems for disease diagnostics, prevention, and treatment. Both researchers and the pharmaceutical industry have shown particular interest in nanotechnology for medical applications with potential benefits for patients. Nanotechnology-based medicine can hurdle both physiological and biological barriers, such as the blood-brain barrier, endothelial barriers, cell membranes, and even nuclear envelopes, achieving both passive and active disease targeting [4]. Nanosized particles are normally composed of thousands of atoms and exhibit unique physical and biochemical properties with high surface area for therapeutic loads such as cancer drugs. However, challenges to nanomedicine exist, such as safety issues, bulk manufacturing issues, and compatibility with the human body of nano-sized drug delivery systems. Meeting these challenges is essential for successful application of drug-loaded nanoparticles (NPs) in the field of pharmaceutics [5].
Different approaches have been taken to conjugate NPs with targeting molecules that specifically bind to the surface markers expressed in various tumors [6], such as prostatespecific membrane antigen (PSMA) in PCa [7]. Prepared NPs are also used in real-time monitoring of treatment by attaching various imaging moieties that help in visualizing the distribution of NPs and the drugs [8]. NPs continue to be investigated for their potential to improve existing therapies and to develop novel therapies. At the length scale of less than 200 nm, NPs with encapsulated therapeutics can be injected intravascularly without concern for embolization. At this scale, NPs have the potential to permeate and traffic through different tissues, bind to cell surface receptors, enter target cells for intracellular delivery of their cargos, and influence the intracellular tracking pathways. Owing to these unique characteristics, a multitude of applications have been identified for NP-mediated drug delivery [9]. NPs can be used as probes with many advantages such as rapid clearance from the bloodstream by macrophages and depending on the size of the NPs renal clearance that helps them to remain in the circulation for a long time [1011]. Indeed, it is important that the NPs have a multivalency effect with more than one type of targeting ligand [1213]. Studies have shown that combined therapy and diagnosis (nanotheranostics), with both imaging and therapeutic capacity, can be achieved [14]. NP composition plays a critical role in drug delivery systems and determines the physicochemical nature of the particle surface for effective delivery. However, some factors of nano-formulated drugs create challenges for batch-to-batch manufacturing, reproducibility, and safety.
A thorough understanding of the therapeutic potential of nanodrugs is necessary for commercialization [15]. The following sections discuss the nanomedicines that have been explored in PCa in vitro and in vivo, respectively.
Nanomedicines have emerged as promising treatments for cancer owing to their noninvasiveness. Live animal imaging technology and advances in nanotechnology are providing new research opportunities in the preclinical and clinical development of nanosized drug carriers in cancer therapy. Nanoparticulate-based medicine has been expanding and has attracted significant interest in recent years, with a primary focus on efficient delivery systems for various chemotherapy drugs [3]. The unique properties of nanomedicines are frequently used to improve the therapeutic value of various water-soluble and insoluble medicinal drugs and bioactive molecules by improving pharmacokinetics behavior, bioavailability, solubility, and retention time [16]. These NP-drug formulations reduce patient expenses and risks of toxicity [4]. Nanoencapsulation of chemotherapy drugs (nanomedicines) increases drug efficacy, specificity, tolerability, and the therapeutic index of corresponding drugs [51718192021].
Several disease-related drugs and bioactive molecules have been successfully encapsulated to improve bioavailability, bioactivity, and controlled delivery [222324]. Nanomedicines are being developed not only for cancer, but also in the area of acquired immunodeficiency syndrome, diabetes, malaria, prion disease, and tuberculosis [252627282930]. Hyaluronic acid has attracted interest for targeted delivery in cancer therapy mainly because of its specificity to bind cancer cells. Another nanomedicine being developed incorporates the well-known anticancer drug cisplatin. Currently, cisplatin is used in various solid tumor treatments [3132]. However, cisplatin has dose-limiting side effects such as neurotoxicity, gastrointestinal disturbance, and nephrotoxicity that limit its clinical use [33]. NP formulations have been developed for efficient delivery of cisplatin [34] and targeting of PCa stem cells by use of CD44 surface marker tagging with hyaluronic acid [3536]. With the use of a drug-induced ionic gelation method, cisplatin glyconanoparticles have been investigated as an efficient drug delivery system targeting PCa stem cells. One group showed that the nanomedicine formulation increases cancer stem cell suppression [37].
A proven concept for drug delivery applications in nanomedicine is the use of carriers such as nanovectors including liposomes, micelles, and dendrimers as well as metallic, ceramic, protein, and polymeric NPs and carbon-based nanostructures [3839]. In particular, carbon nanotubes possess unique features for biomedical applications, including the possibility of chemical functionalization with drugs and their internalization by various cell types through endocytosis and passive diffusion across cellular membranes. Carbon nanotubes have been shown to efficiently transport and release various anticancer drugs [40414243]. With the use of a carbon nanotube-based approach, the natural compound catechin was recently explored as a potential therapeutic nanomedicine for PCa treatment. Catechin is in the family of polyphenolic flavonoids, and catechin and its derivatives (2)-epigallocatechin-3-gallate, (2)-epigallocatechin, (2)-epicatechin-3-gallate, and (2)-epicatechin inhibit cancer cell proliferation, suppress tumor growth and metastasis, and sensitize tumors to various chemotherapy drugs and radiotherapy [444546]. Recently, a study showed that biocompatible catechin-loaded and gelatin-conjugated carbon nanotubes have good anticancer properties with the potential for targeting prostate PCa stem cells in combination with x-ray irradiation [47].
The various chemotherapy drugs currently in use have some drawbacks. For example, the semisynthetic chemotherapy drug known as docetaxel, which is used alone and in combination for various tumors, has limitations including poor aqueous solubility and low oral bioavailability. Moreover, docetaxel has intolerable side effects such as acute hypersensitivity reactions, fluid retention, neurotoxicity, and febrile neutropenia [4849]. To overcome these disadvantages of docetaxel, nanomedicine has been explored for passive and active targeting by enhancing permeability and retention (EPR) effect, avoidance of the reticuloendothelial system, prolonged blood circulation time, controlled release of drug, and providing an opportunity for surface modification for active drug targeting [5051]. To enhance the permeability and bioavailability of docetaxel, one researcher used D-α-tocopheryl polyethylene glycol succinate (TPGS, or Vit E TPGS), a Food and Drug Administration-approved adjuvant for drug delivery, and showed that cyclic peptide (cRGDfK) can be used to conjugate succinoyl TPGS nanomicelles for docetaxel delivery in a successful PCa-targeted drug delivery system [52].
Another interesting nanoparticulate system for cancer treatment is magnetic iron oxide NP clusters, which have been shown as promising drug delivery vehicles for both diagnostic and therapeutic applications. Magnetic NP clusters have been shown to be stable in a biological circulation system, to readily interact with cells or other biological units of interest, and to be capable of releasing the drug once the selected targeting is realized. In addition, the magnetic NP clusters are biocompatible, have been proven to have no toxicity in vivo, and have shown efficient contrast in magnetic resonance imaging (MRI) [53]. Magnetic NP clusters are excellent near-infrared photothermal mediators in the combination of photothermal therapy and chemotherapy. Recently, doxorubicin (DOX) when loaded into magnetic NP clusters achieved greater PCa cell cytotoxicity owing to both cluster-mediated photothermal ablation and cytotoxicity of light-triggered DOX release. Compared with photothermal therapy or chemotherapy alone, the chemophotothermal therapy approach with DOX in magnetic NP clusters showed synergistically higher therapeutic efficacy both in vitro and in vivo for treatment of the PCa cell line PC3 [54].
Natural gum arabic glycol protein has recently been explored for homogeneous dose distribution and efficient emission. Researchers have reported that NPs coated with gum arabic are stable for in vivo applications. One study showed that intralesional injection of gum Arabic-coated radioactive gold NPs resulted in minimal short-term systemic toxicity in a naturally occurring large animal model of PCa [55]. To achieve better therapeutic efficacy, the use of liposomal micelles to encapsulate dexamethasone was recently reported for sustained, antitumor effects versus free dexamethasone in metastatic bone disease from human PCa. These studies showed that intravenously administered liposomes localized efficiently to bone metastases in vivo and that treatment of established bone metastases with (liposomal) dexamethasone resulted in a significant inhibition of tumor growth up to 26 days after the initiation of treatment [56]. Furthermore, 1.0-mg/kg liposomal dexamethasone significantly outperformed 1.0-mg/kg free dexamethasone and was found to be well tolerated at clinically relevant dosages. Delivery of liposomal-loaded glucocorticoid dexamethasone was thus proposed for future clinical evaluation as a promising new treatment option for advanced, metastatic PCa [56].
In contrast to the overexpression of a membrane lectin, expression of the cation-independent mannose 6-phosphate receptor (M6PR) was specifically demonstrated in PCa cell lines and tissues. M6PR was proposed as a new, efficient target and alternative biomarker to PSMA for nanomedicine applications. M6PR is a ubiquitous receptor involved in several biological functions, mainly in addressing enzymes to lysosomes [575859]. In one study, a mannose-6-phosphate analogue was synthesized and grafted onto the surface of functionalized mesoporous silica NPs as a theranostic therapy for PCa. Mesoporous silica NPs can be used for targeting, imaging, and photodynamic therapy. Therefore, M6PR appears as a promising new target for nanomedicine applications in PCa, particularly for noninvasive and personalized therapy of small-sized prostate tumors [60].
In the area of novel drug inhibitors, histone deacetylase inhibitors (HDACIs) do not have significantly better outcomes in solid malignancies than current standard therapies [61]. Moreover, the efficacy of the first-generation HDACIs was limited in solid tumor indications owing to their suboptimal potency for specific HDAC enzymes and transient induction of histone acetylation in tumor tissue [62]. Considering the several drawbacks of HDACIs, nanomedicine applications have been designed with novel NP formulations of HDACIs, vorinostat and quisinostat, which release the drug in a slow and controlled fashion. The NPs vorinostat and quisinostat demonstrated higher therapeutic efficacy than small-molecule HDACIs in chemoradiotherapy in murine models of PCa. Vorinostat and quisinostat are promising cancer therapeutics with the potential to significantly improve chemoradiotherapy [63].
The chemotherapy drug docetaxel, a second-generation taxoid, has antimitotic chemotherapeutic properties and has been used for a variety of cancers, especially PCa. In response to drawbacks such as docetaxel solubility, toxicity issues, and inaccurate tumor targeting, researchers have successfully fabricated docetaxel as a nanomedicine for sustained drug release by use of a copolymer-based poly(styrene)-b-poly(DL-lactide) (PS-PDLLA) for intravenous injection. Interestingly, the carrier copolymer did not induce any serious cytotoxicity in PC3 cells. Moreover, improved inhibition of cancer cell growth was shown in the group treated with PS-PDLLA/docetaxel compared with the control group. Decreased in vivo drug clearance, elevated systemic exposure, and prolonged circulation of the drug were verified in a pharmacokinetic study in rats, which further supports the potential use of nanomedicines in PCa therapy [64].
In a similar study, researchers developed a novel nanoplatform based on N-(2-hydroxypropyl) methacrylamide (HPMA) copolymers that allows covalent bonding of two chemotherapeutics acting via different anticancer mechanisms and that can enter target cells by receptor-mediated endocytosis. In that study, DOX and 5-fluorouracil (5-Fu) were covalently conjugated to a nanosized HPMA copolymer via a pH-sensitive hydrazone bond and an enzymatically degradable oligopeptide Gly-Phe-Leu-Gly sequence. The conjugate was decorated with galectin-3 targeting peptide G3-C12 [P-(G3-C12)-DOX-Fu]. Interestingly, these two drugs showed similar release profiles in vitro, suggesting synergistic effects in the co-delivery system. Studies in the cellular system showed that overexpression of galectin-3 in human PC3 prostate carcinoma cells treated with a combination of the drug P-(G3-C12)-DOX-Fu surprisingly exhibited comparable cytotoxicity to free DOX at high concentrations by increasing cell internalization and exerting synergistic genotoxic effects of cell cycle arrest, caspase-3 activation, and DNA damage. In mice bearing PC3 tumor xenografts, the use of tumor-targeting ligand substantially enhanced the intracellular delivery of P-(G3-C12)-DOX-Fu with no adverse effects. Thus, potential exists for synergistic combination therapy using targeted nanomedicines for efficient treatment of PCa [65].
Like other imaging modalities such as computed tomography, transurethral ultrasound, and nuclear imaging, MRI cannot adequately detect small tumors [66]. Improvements in tumor imaging technologies are urgently required for early detection of disease, staging, and realtime assessment of response to therapy in PCa patients. Similar to other metal nanocarriers for nanomedicine, iron oxide magnetic NPs have emerged as powerful contrast agents for MRI [67]. One research group developed iron oxide magnetic NPs to enhance MRI of PCa with the PSMAtargeting antibody, J591, via a 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol) (PEGDSPE) linkage. In this preclinical model, PSMA-targeted magnetic NPs effectively enhanced MRI of PCa [68].
Another interesting area of research on phototherapy is the quantum dot (QD) technique, and numerous studies have been done with QD-based fluorophores with high photostability for diagnostics and biomedical assays. QD-based systems can achieve cost-effective antigen detection. Recently, researchers designed a new multiplexed diagnostic system based on QD-encoded beads for simultaneous detection of free and total PSA for ultrasensitive, accurate, early PCa diagnosis [69].
In contrast to the nano-micelle area, other research groups have developed a carrier system for castrate-resistant PCa by using raloxifene-loaded poly(styrene-co-maleic acid) (SMA) micelles (SMA-raloxifene) to improve the biodistribution of raloxifene and increase its anticancer efficacy. The effect of SMA-raloxifene on apoptosis, reduction of cell proliferation, migration, and invasion of castration-resistant PCa (CRPC) cells was superior compared with free drug in vitro [70]. In addition, SMA-raloxifene induced changes in expression and localization of the estrogen receptors, as well as downstream effectors associated with cell proliferation and survival [70]. This study examined the cellular internalization and efflux of SMA-raloxifene compared with free raloxifene. The biodistribution and anticancer efficacy of SMA-raloxifene were then compared with free raloxifene in a CRPC mouse xenograft model [71].
In the area of radiation therapy for prostate tumors, researchers have developed gold NPs that can be targeted by using goserelin-conjugated gold nanorods. In this approach, megavoltage radiosensitization in vivo using tumor goserelin-targeted gold NPs showed independent mechanisms of tumor accumulation in vivo (EPR effect as a consequence of leaky tumor vasculature and active uptake by target cells). In combination with megavoltage radiation therapy, this led to delays in tumor growth in mice with heterotopic PCa. More importantly, significant radiosensitization to megavoltage radiation was not observed with unconjugated gold NPs despite their intratumoral concentration being only 3 folds less than that of conjugated gold NPs. This suggests that radiosensitization is improved by active targeting that leads to cellular internalization of NPs and the consequent increase in ionization density within the cytoplasm, rather than merely passive accumulation of NPs in the perivascular space by EPR. This report concluded that conjugation of NPs to tumor-specific antigens, which promotes internalization within cancer cells, causes radiosensitization and that goserelin-conjugated gold NPs can be effective PCa radiosensitizers when used with megavoltage radiation [72].
Another strategy being developed is to combine a chemotherapy drug and a natural drug as a hybrid nanomedicine for metastatic CRPC. One such study used lipid-polymer hybrid NPs to co-encapsulate DTX and curcumin (CUR). In mice bearing PC3 tumor xenografts, the DTX-CUR lipid-polymer NPs synergistically inhibited tumor growth to a greater extent with no adverse effects. Nanomedicine offers great promise for dual drug delivery to PCa cells, with the potential for synergistic combination therapy [73]
In summary, interesting nanomedicines have been developed for PCa in recent years. These namomedicines need to be further evaluated in higher animals in terms of their safety and toxicity profiles.
Gene therapy offers a solution to controlled and specific delivery of DNA, RNA, and protein to cells. Chitosan-based carriers are nonviral vectors that have gained popularity as a safe delivery system for gene materials including plasmid DNA, oligonucleotides, and small inhibitory RNA (siRNA). Chitosan has beneficial qualities such as low toxicity, low immunogenicity, and excellent biocompatibility [7475]. Our group reported a preliminary investigation with siRNA targeted to AGR2 conjugated with chitosan to form NPs. Secretion of AGR2 plays a predominant role in PCa progression and metastasis. The results of scanning electron microscopy and gel electrophoresis showed efficient condensation of siRNA for AGR2 [76]. To improve the transfection efficiency of the AGR2 siRNA, the complexes were tested in the human PC3 prostate cell line, which overexpresses AGR2 protein. In PC3 cells, the chitosan and siRNA AGR2 complex was found to be efficient and nontoxic to overall cells by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Thus, chitosan NPs have been shown to be a safe vector system for efficient gene delivery in cancer. Our study proposed further development of chitosan-based formulations for the delivery of AGR2 siRNA for clinical applications, which may represent a promising approach in PCa treatment [76].
Interestingly, another delivery strategy for siRNA in a vector involved coupling stearic acid to the N-terminus of HHHPKPKRKV. STR-HK formed stable complexes with siRNA via noncovalent interactions and mediated efficient delivery of siRNA to PC3 cells with minimal cytotoxicity compared with a gene-silencing chemical transfection agent like lipofectamine. These results proved that STR-HK is a promising vector for siRNA delivery [77]. In a similar fashion, research also demonstrated that intratumoral injections of siRNAs loaded on biodegradable chitosan NPs led to a downregulation of RXFP1 receptor expression and a dramatic reduction in tumor growth [78]. In the xenograft models treated with siRNA against RXFP1, the smaller tumor size was associated with decreased cell proliferation and increased apoptosis, which suggests that the suppression of RLN/RXFP1 might have potential therapeutic benefits in PCa [78]. Another interesting report showed that dicer complex of siRNA (dsiRNA), when delivered using a structurally flexible triethanolamine-core poly(amidoamine) dendrimer as the nanocarrier, gives rise to a much greater RNAi response than that with conventional siRNA. Dendrimer/dsiRNA complexes with a dual targeting peptide simultaneously promoted cancer cell targeting by interacting with integrins and cell penetration via the interaction with neuropilin-1 receptors, which led to improved gene silencing and anticancer activity. These findings suggest therapeutic applications of RNAi using dendrimer nanovector-based targeted delivery [79].
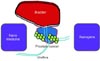
The role of nanomedicines and nanogene delivery in theranostics approaches for PCa are summarized in Fig. 1. Preclinical safety guidelines still need to be addressed.
This review has highlighted nanomedicine and nanogene therapy research in association with nanotechnology concepts for modern medicine, especially for improving the therapeutic outcomes of cancer. This review has also presented recent developments in nanomedicine for future clinical applications. Nanomedicine can effectively deliver medicine specifically to prostate tumor sites (Table 1). As the technology is perfected, nanomedicines can be used in the future in benign prostatic hyperplasia at the level of expression and location of proteins [80]. As image-guiding techniques such as magnetic iron oxide NPs (Fe3O4 NPs) are perfected, image-guided radiation therapy can be used for PCa [81]. Moreover, application of nanotechnology to extraperitoneal laparoscopic radical prostatectomy [82] can enable patients to have a better quality of life and improve the survival rate in the near future.
Figures and Tables
Table 1
Tabular column indicating the nanomedicine and nanogene delivery for prostate cancer
| Area of prostate nanomedicine | Delivery system | References |
|---|---|---|
| Nanomedicine delivery | Magnetic nanocomplex-doxorubicin | Zhang et al. [15] |
| cis Dichlorodiamminoplatinum (II) glyconanoparticles | Jafari Malek et al. [37] | |
| Carbon nanotubes catechin | Castro Nava et al. [47] | |
| Succinoyl-tocopheryl polyethylene glycol succinate nanomicelles doxorubicin | Kulhari et al. [52] | |
| Gum arabic-coated radioactive gold nanoparticles (GA-198AuNPs) | Axiak-Bechtel et al. [55] | |
| Liposomal-dexamethasone | Kroon et al. [56] | |
| Mesoporous silica nanoparticles-mannose-6-phosphate receptor | Vaillant et al. [60] | |
| Histone deacetylase inhibitors | Yao et al. [33] | |
| N-(2-hydroxypropyl) methacrylamide | Yang et al. [65] | |
| Poly(styrene)-b-poly(DL-lactide) copolymer-based (NPs)-delivery of docetaxel NPs | Lee et al. [64] | |
| Iron oxide magnetic nanoparticles-prostate-specific membrane antigen | Tse et al. [68] | |
| Quantum dot-prostate-specific antigen | Brazhnik et al. [69] | |
| Raloxifene nanomicelles | Pritchard et al. [71] | |
| Goserelin-conjugated gold nanorods | Wolfe et al. [72] | |
| Docetaxel and curcumin coencapsulated | Yan et al. [73] | |
| Nano gene delivery | Chitosan- Suppression of relaxin receptor relaxin family peptide receptor 1 | Feng et al. [78] |
| Chitosan-AGR2 small inhibitory RNA | Mohandas et al. [76] | |
| Stearylated cationic peptide-small inhibitory RNA | Chen et al. [77] | |
| Dicer-substrate small inhibitory RNAs-dendrimers | Liu et al. [79] |
ACKNOWLEDGMENTS
The author acknowledges Research Institute of Biomedical Sciences, Chonnam National University Medical School and Chonnam National University for infrastructure support.
References
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012; 62:10–29.
2. Han HH, Park JW, Na JC, Chung BH, Kim CS, Ko WJ. Epidemiology of prostate cancer in South Korea. Prostate Int. 2015; 3:99–102.
3. Weissleder R. A clearer vision for in vivo imaging. Nat Biotechnol. 2001; 19:316–317.
4. NanoMarkets white paper. Nano-enabled drug delivery systems market: the impact of nanotechnology in drug delivery: global developments, market analysis, and future prospects [Internet]. Sterling (VA): NanoMarkets, LC;2004. cited 2015 Sep 11. Available from: http://www.pharmamanufacturing.com/assets/Media/MediaManager/NanoMarkets_Drug_Delivery_122004.pdf.
5. Safra T, Muggia F, Jeffers S, Tsao-Wei DD, Groshen S, Lyass O, et al. Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol. 2000; 11:1029–1033.
6. Lakshmanan VK, Snima KS, Bumgardner JD, Nair SV, Jayakumar R. Chitosan-based nanoparticles in cancer therapy. Adv Polym Sci. 2011; 243:55–92.
7. Chen Z, Penet MF, Nimmagadda S, Li C, Banerjee SR, Winnard PT Jr, et al. PSMA-targeted theranostic nanoplex for prostate cancer therapy. ACS Nano. 2012; 6:7752–7762.
8. Cherian AM, Nair SV, Lakshmanan VK. The role of nanotechnology in prostate cancer theranostic applications. J Nanosci Nanotechnol. 2014; 14:841–852.
9. Hickey JW, Santos JL, Williford JM, Mao HQ. Control of polymeric nanoparticle size to improve therapeutic delivery. J Control Release. 2015; 219:536–547.
10. Zhou C, Long M, Qin Y, Sun X, Zheng J. Luminescent gold nanoparticles with efficient renal clearance. Angew Chem Int Ed Engl. 2011; 50:3168–3172.
11. Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, et al. Renal clearance of quantum dots. Nat Biotechnol. 2007; 25:1165–1170.
12. Lee JH, Lee K, Moon SH, Lee Y, Park TG, Cheon J. All-in-one target-cell-specific magnetic nanoparticles for simultaneous molecular imaging and siRNA delivery. Angew Chem Int Ed Engl. 2009; 48:4174–4179.
13. Cheon J, Lee JH. Synergistically integrated nanoparticles as multimodal probes for nanobiotechnology. Acc Chem Res. 2008; 41:1630–1640.
14. Mura S, Couvreur P. Nanotheranostics for personalized medicine. Adv Drug Deliv Rev. 2012; 64:1394–1416.
15. Zhang L, Zhang N. How nanotechnology can enhance docetaxel therapy. Int J Nanomedicine. 2013; 8:2927–2941.
16. Shenoy DB, Amiji MM. Poly(ethylene oxide)-modified poly(epsilon-caprolactone) nanoparticles for targeted delivery of tamoxifen in breast cancer. Int J Pharm. 2005; 293:261–270.
17. Schroeder U, Sommerfeld P, Ulrich S, Sabel BA. Nanoparticle technology for delivery of drugs across the blood-brain barrier. J Pharm Sci. 1998; 87:1305–1307.
18. Raghuvanshi RS, Katare YK, Lalwani K, Ali MM, Singh O, Panda AK. Improved immune response from biodegradable polymer particles entrapping tetanus toxoid by use of different immunization protocol and adjuvants. Int J Pharm. 2002; 245:109–121.
19. Kreuter J. Influence of chronobiology on the nanoparticle-mediated drug uptake into the brain. Pharmaceutics. 2015; 7:3–9.
20. Fassas A, Buffels R, Kaloyannidis P, Anagnostopoulos A. Safety of high-dose liposomal daunorubicin (daunoxome) for refractory or relapsed acute myeloblastic leukaemia. Br J Haematol. 2003; 122:161–163.
21. Ding Y, Wang Y, Opoku-Damoah Y, Wang C, Shen L, Yin L, et al. Dual-functional bio-derived nanoparticulates for apoptotic antitumor therapy. Biomaterials. 2015; 72:90–103.
22. Budhian A, Siegel SJ, Winey KI. Production of haloperidol-loaded PLGA nanoparticles for extended controlled drug release of haloperidol. J Microencapsul. 2005; 22:773–785.
23. Gomez-Gaete C, Tsapis N, Besnard M, Bochot A, Fattal E. Encapsulation of dexamethasone into biodegradable polymeric nanoparticles. Int J Pharm. 2007; 331:153–159.
24. Cheng Q, Feng J, Chen J, Zhu X, Li F. Brain transport of neurotoxin-I with PLA nanoparticles through intranasal administration in rats: a microdialysis study. Biopharm Drug Dispos. 2008; 29:431–439.
25. Mu L, Feng SS. A novel controlled release formulation for the anticancer drug paclitaxel (Taxol): PLGA nanoparticles containing vitamin E TPGS. J Control Release. 2003; 86:33–48.
26. Coester C, Kreuter J, von Briesen H, Langer K. Preparation of avidin-labelled gelatin nanoparticles as carriers for biotinylated peptide nucleic acid (PNA). Int J Pharm. 2000; 196:147–149.
27. Damge C, Maincent P, Ubrich N. Oral delivery of insulin associated to polymeric nanoparticles in diabetic rats. J Control Release. 2007; 117:163–170.
28. Date AA, Joshi MD, Patravale VB. Parasitic diseases: liposomes and polymeric nanoparticles versus lipid nanoparticles. Adv Drug Deliv Rev. 2007; 59:505–521.
29. Calvo P, Gouritin B, Brigger I, Lasmezas C, Deslys J, Williams A, et al. PEGylated polycyanoacrylate nanoparticles as vector for drug delivery in prion diseases. J Neurosci Methods. 2001; 111:151–155.
30. Ahmad Z, Pandey R, Sharma S, Khuller GK. Alginate nanoparticles as antituberculosis drug carriers: formulation development, pharmacokinetics and therapeutic potential. Indian J Chest Dis Allied Sci. 2006; 48:171–176.
31. Hogberg T, Glimelius B, Nygren P. SBU-group. Swedish council of technology assessment in health care: a systematic overview of chemotherapy effects in ovarian cancer. Acta Oncol. 2001; 40:340–360.
32. Hundahl SA. Surgical quality in trials of adjuvant cancer therapy. J Surg Oncol. 2002; 80:177–180.
33. Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci. 2007; 334:115–124.
34. Avgoustakis K, Beletsi A, Panagi Z, Klepetsanis P, Karydas AG, Ithakissios DS. PLGA-mPEG nanoparticles of cisplatin: in vitro nanoparticle degradation, in vitro drug release and in vivo drug residence in blood properties. J Control Release. 2002; 79:123–135.
35. Nateghian N, Goodarzi N, Amini M, Atyabi F, Khorramizadeh MR, Dinarvand R. Biotin/Folate decorated human serum albumin-nanoparticles of docetaxel: comparison of chemically conjugated nanostructures and physically loaded nanoparticles for targeting of breast cancer. Chem Biol Drug Des. 2015; Jul. 28. [Epub]. DOI: 10.1111/cbdd.12624.
36. Goodarzi N, Ghahremani MH, Amini M, Atyabi F, Ostad SN, Shabani Ravari N, et al. CD44-targeted docetaxel conjugate for cancer cells and cancer stem-like cells: a novel hyaluronic acidbased drug delivery system. Chem Biol Drug Des. 2014; 83:741–752.
37. Jafari Malek S, Khoshchehreh R, Goodarzi N, Khoshayand MR, Amini M, Atyabi F, et al. cis-Dichlorodiamminoplatinum (II) glyconanoparticles by drug-induced ionic gelation technique targeted to prostate cancer: preparation, optimization and in vitro characterization. Colloids Surf B Biointerfaces. 2014; 122:350–358.
38. Rastogi V, Yadav P, Bhattacharya SS, Mishra AK, Verma N, Verma A, et al. Carbon nanotubes: an emerging drug carrier for targeting cancer cells. J Drug Deliv. 2014; 2014:670815.
39. Drbohlavova J, Chomoucka J, Adam V, Ryvolova M, Eckschlager T, Hubalek J, et al. Nanocarriers for anticancer drugs: new trends in nanomedicine. Curr Drug Metab. 2013; 14:547–564.
40. Danhier F, Feron O, Preat V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010; 148:135–146.
41. Lacerda L, Russier J, Pastorin G, Herrero MA, Venturelli E, Dumortier H, et al. Translocation mechanisms of chemically functionalised carbon nanotubes across plasma membranes. Biomaterials. 2012; 33:3334–3343.
42. Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000; 65:271–284.
43. Wong BS, Yoong SL, Jagusiak A, Panczyk T, Ho HK, Ang WH, et al. Carbon nanotubes for delivery of small molecule drugs. Adv Drug Deliv Rev. 2013; 65:1964–2015.
44. Weng CJ, Yen GC. Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities. Cancer Metastasis Rev. 2012; 31:323–351.
45. Tang SN, Singh C, Nall D, Meeker D, Shankar S, Srivastava RK. The dietary bioflavonoid quercetin synergizes with epigal-locathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition. J Mol Signal. 2010; 5:14.
46. Khan N, Bharali DJ, Adhami VM, Siddiqui IA, Cui H, Shabana SM, et al. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis. 2014; 35:415–423.
47. Castro Nava A, Cojoc M, Peitzsch C, Cirillo G, Kurth I, Fuessel S, et al. Development of novel radiochemotherapy approaches targeting prostate tumor progenitor cells using nanohybrids. Int J Cancer. 2015; 137:2492–2503.
48. Cho K, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008; 14:1310–1316.
49. Baker J, Ajani J, Scotte F, Winther D, Martin M, Aapro MS, et al. Docetaxel-related side effects and their management. Eur J Oncol Nurs. 2009; 13:49–59.
50. Sultana S, Khan MR, Kumar M, Kumar S, Ali M. Nanoparticles-mediated drug delivery approaches for cancer targeting: a review. J Drug Target. 2013; 21:107–125.
51. Farrell D, Ptak K, Panaro NJ, Grodzinski P. Nanotechnology-based cancer therapeutics--promise and challenge--lessons learned through the NCI Alliance for Nanotechnology in Cancer. Pharm Res. 2011; 28:273–278.
52. Kulhari H, Pooja D, Shrivastava S, Telukutala SR, Barui AK, Patra CR, et al. Cyclic-RGDfK peptide conjugated succinoyl-TPGS nanomicelles for targeted delivery of docetaxel to integrin receptor over-expressing angiogenic tumours. Nanomedicine. 2015; 11:1511–1520.
53. Shen S, Wang S, Zheng R, Zhu X, Jiang X, Fu D, et al. Magnetic nanoparticle clusters for photothermal therapy with near-infrared irradiation. Biomaterials. 2015; 39:67–74.
54. Li M, Lin F, Lin Y, Peng W. Extracellular polysaccharide from Bordetella species reduces high glucose-induced macrophage apoptosis via regulating interaction between caveolin-1 and TLR4. Biochem Biophys Res Commun. 2015; 466:748–754.
55. Axiak-Bechtel SM, Upendran A, Lattimer JC, Kelsey J, Cutler CS, Selting KA, et al. Gum arabic-coated radioactive gold nanoparticles cause no short-term local or systemic toxicity in the clinically relevant canine model of prostate cancer. Int J Nanomedicine. 2014; 9:5001–5011.
56. Kroon J, Buijs JT, van der Horst G, Cheung H, van der Mark M, van Bloois L, et al. Liposomal delivery of dexamethasone attenuates prostate cancer bone metastatic tumor growth in vivo. Prostate. 2015; 75:815–824.
57. Gary-Bobo M, Nirde P, Jeanjean A, Morere A, Garcia M. Mannose 6-phosphate receptor targeting and its applications in human diseases. Curr Med Chem. 2007; 14:2945–2953.
58. Ghosh P, Dahms NM, Kornfeld S. Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol. 2003; 4:202–212.
59. Grubb JH, Vogler C, Sly WS. New strategies for enzyme replacement therapy for lysosomal storage diseases. Rejuvenation Res. 2010; 13:229–236.
60. Vaillant O, El Cheikh K, Warther D, Brevet D, Maynadier M, Bouffard E, et al. Mannose-6-phosphate receptor: a target for theranostics of prostate cancer. Angew Chem Int Ed Engl. 2015; 54:5952–5956.
61. Chueh AC, Tse JW, Togel L, Mariadason JM. Mechanisms of histone deacetylase inhibitor-regulated gene expression in cancer cells. Antioxid Redox Signal. 2015; 23:66–84.
62. Arts J, King P, Marien A, Floren W, Belien A, Janssen L, et al. JNJ-26481585, a novel "second-generation" oral histone deacetylase inhibitor, shows broad-spectrum preclinical anti-tumoral activity. Clin Cancer Res. 2009; 15:6841–6851.
63. Wang EC, Min Y, Palm RC, Fiordalisi JJ, Wagner KT, Hyder N, et al. Nanoparticle formulations of histone deacetylase inhibitors for effective chemoradiotherapy in solid tumors. Biomaterials. 2015; 51:208–215.
64. Lee JY, Kim JS, Cho HJ, Kim DD. Poly(styrene)-b-poly(DL-lactide) copolymer-based nanoparticles for anticancer drug delivery. Int J Nanomedicine. 2014; 9:2803–2813.
65. Yang Q, Yang Y, Li L, Sun W, Zhu X, Huang Y. Polymeric nano-medicine for tumor-targeted combination therapy to elicit synergistic genotoxicity against prostate cancer. ACS Appl Mater Interfaces. 2015; 7:6661–6673.
66. Taneja SS. Imaging in the diagnosis and management of prostate cancer. Rev Urol. 2004; 6:101–113.
67. Liu G, Gao J, Ai H, Chen X. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small. 2013; 9:1533–1545.
68. Tse BW, Cowin GJ, Soekmadji C, Jovanovic L, Vasireddy RS, Ling MT, et al. PSMA-targeting iron oxide magnetic nanoparticles enhance MRI of preclinical prostate cancer. Nanomedicine (Lond). 2015; 10:375–386.
69. Brazhnik K, Sokolova Z, Baryshnikova M, Bilan R, Efimov A, Nabiev I, et al. Quantum dot-based lab-on-a-bead system for multiplexed detection of free and total prostate-specific antigens in clinical human serum samples. Nanomedicine. 2015; 11:1065–1075.
70. Taurin S, Nehoff H, van Aswegen T, Rosengren RJ, Greish K. A novel role for raloxifene nanomicelles in management of castrate resistant prostate cancer. Biomed Res Int. 2014; 2014:323594.
71. Pritchard T, Rosengren RJ, Greish K, Taurin S. Raloxifene nanomicelles reduce the growth of castrate-resistant prostate cancer. J Drug Target. 2015; Sep. 16. 1. [Epub]. DOI: 10.3109/1061186X.2015.1086360.
72. Wolfe T, Chatterjee D, Lee J, Grant JD, Bhattarai S, Tailor R, et al. Targeted gold nanoparticles enhance sensitization of prostate tumors to megavoltage radiation therapy in vivo. Nanomedicine. 2015; 11:1277–1283.
73. Yan J, Wang Y, Zhang X, Liu S, Tian C, Wang H. Targeted nanomedicine for prostate cancer therapy: docetaxel and curcumin co-encapsulated lipid-polymer hybrid nanoparticles for the enhanced anti-tumor activity in vitro and in vivo. Drug Deliv. 2015; Jul. 27. 1. [Epub]. DOI: 10.3109/10717544.2015.1069423.
74. Guang Liu W, De Yao K. Chitosan and its derivatives--a promising non-viral vector for gene transfection. J Control Release. 2002; 83:1–11.
75. Shu XZ, Zhu KJ. The influence of multivalent phosphate structure on the properties of ionically cross-linked chitosan films for controlled drug release. Eur J Pharm Biopharm. 2002; 54:235–243.
76. Mohandas A, Snima KS, Jayakumar R, Lakshmanan VK. Chitosan based AGR2 siRNA nanoparticle delivery system for prostate cancer cells. J Chitin Chitosan Sci. 2013; 1:161–165.
77. Chen B, Pan R, Askhatova D, Chen P. Effective small interfering RNA delivery in vitro via a new stearylated cationic peptide. Int J Nanomedicine. 2015; 10:3303–3314.
78. Feng S, Agoulnik IU, Truong A, Li Z, Creighton CJ, Kaftanovskaya EM, et al. Suppression of relaxin receptor RXFP1 decreases prostate cancer growth and metastasis. Endocr Relat Cancer. 2010; 17:1021–1033.
79. Liu X, Liu C, Chen C, Bentobji M, Cheillan FA, Piana JT, et al. Targeted delivery of Dicer-substrate siRNAs using a dual targeting peptide decorated dendrimer delivery system. Nanomedicine. 2014; 10:1627–1636.
80. Hwang I, Jung SI, Hwang EC, Song SH, Lee HS, Kim SO, et al. Expression and localization of aquaporins in benign prostate hyperplasia and prostate cancer. Chonnam Med J. 2012; 48:174–178.
81. Cho JK, Song HJ, Song HC, Kim KJ, Lee YG. Application of image-guided radiation therapy (IGRT) with gold markers in prostate cancer. Chonnam Med J. 2009; 45:182–187.
82. Chung HS, Yun BH, Ki HC, Na SW, Hwang EC, Im CM, et al. Extraperitoneal laparoscopic radical prostatectomy: clinical experience and learning curve with 103 cases. Chonnam Med J. 2010; 46:170–176.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download