Abstract
We present a 61-year-old man who was diagnosed with synchronous prostate cancer and suspicious renal cell carcinoma of the right kidney, treated with combined Retzius-sparing robot-assisted radical prostatectomy (RS-RARP) and robot-assisted partial nephrectomy (RAPN). The combined approach using RS-RARP and RAPN is technically feasible and safe surgical option for treatment of concomitant prostate cancer and suspicious renal cell carcinoma.
The incidence of second primary cancer detection in patients with prostate cancer (PCa) undergoing cross-sectional imaging for staging workup is 1.5% [1]. Subsequently, reports of concurrent PCa and renal masses are appearing in the literature. Since the introduction of robotic surgery, experienced surgeons have extended its use from simple to challenging cases. Robotic systems have given the surgeon the ability to surpass previous obstacles related to disease management. Recently, Patel et al. [2] reported the first combined robot-assisted partial nephrectomy (RAPN) and robot-assisted radical prostatectomy (RARP) in 59-year-old man with synchronous PCa and renal cell carcinoma (RCC), and they demonstrated the feasibility of performing concurrent upper and lower urinary tract robotic-assisted surgeries by reusing port incisions to decrease surgical morbidity.
To the best of our knowledge, this is the first reported case which utilized the Retzius-sparing RARP (RS-RARP) in the combined approach for the treatment of synchronous PCa and RCC.
A 61-year-old male patient with concurrent prostatic adenocarcinoma and an enhancing right-sided renal mass was referred to our robotic oncology center for evaluation and treatment. He had no comorbidity or previous surgery. Patient's sexual health inventory for men questionnaire score was 14, body mass index was 25.4 kg/m2, and digital rectal examination was normal. Serum creatinine and prostate-specific antigen were 1.0 mg/dL and 6.7 ng/mL, respectively. The prostatic biopsy revealed adenocarcinoma Gleason score (GS) 6 (3+3), in one core with core percentage volume of 50%. On pelvic magnetic resonance imaging, the prostate volume was 29 mL, and there was no typical cancerous lesion in the prostate and no evidence of extracapsular extension, seminal vesicle invasion or pelvic lymph nodes enlargement. A contrast-enhanced computed tomography scan of the abdomen and pelvis showed 1.6-cm enhancing endophytic mass in the posteromedial portion of right kidney suggestive of RCC. Bone scan showed no metastasis. The patient underwent RS-RARP and selectiveclamp RAPN in the same setting. Based on Partin's table our patient has 80% probability of organ confined disease and 0% computed risk for lymph node metastasis [3]. Pelvic lymph node dissection may be omitted for patients when predicted probability of lymph node metastasis based on clinical information is less than 3% [4].
We used the DaVinci-Si Robotic Surgical System (Intuitive Surgical, Sunnyvale, CA, USA) to perform both procedures.
RS-RARP was performed first followed by right-sided RAPN, because our team carries out RS-RARP in short operative time (less than 2 hours), and with minimal blood loss, we chose to perform robotic prostatectomy first for safety issues. Notably, we took full precautions to manage any intraoperative complications that may occur during surgery and to deal with any unexpected difficulties.
For RS-RARP we inserted a supraumbilical 12-mm camera port (No. 1), three working 8-mm ports (No. 2, 3, and 4) for robotic instruments and 2 assistant 5-mm (No. 6) and 12-mm (No. 7) ports. After we finished RS-RARP, we undocked all ports, draped the patient abdomen and sutured port (No. 4 and 7). For RAPN we reused port (No. 1) as 12-mm camera port, ports (No. 2 and 3) as 8-mm working ports and port (No. 6) for liver retraction. We placed 2 additional ports, 8-mm working port (No. 5) and 12-mm assistant port (No. 8). The assistant 8-mm port (No. 8) was triangulated with the camera port and port (No. 2) to allow easy renal access without interference with the camera and robotic arms. In order to have a capacious working space during RAPN and to avoid collision between robotic arms, we shifted ports (No. 2 and 3) to a more caudal location than its standard usual. In reference to our previously reported study on our initial experience with RS-RARP, we maintained the same 8-mm distance from camera port to left arm and 7-cm distance to right arm from camera port [5]. In our current study, arms 2 and 3 was kept within 8-cm distance apart from each other with approximately 1 cm caudal than originally described position from our previous report. The incision of the supraumbilical camera port was extended to 4-cm incision to retrieve the prostate and renal specimens out (Fig. 1; Video clip, Supplementary material).
The surgical steps included mobilization of the colon and posterior peritoneal incision. The vas deferens is mobilized and clipped bilaterally. Athermal dissection of seminal vesicles was carried out. Lateral dissection of the lateral prostatic pedicles was commenced. Dissection of the prostate was performed circumferentially towards the antero-lateral aspect of the prostate and from the bladder neck distally towards the apex of the prostate. An intrafascial plane posterior to the prostate was developed using blunt and sharp dissection. Bladder neck was dissected with sharp dissection, the detrusor muscles at the bladder neck are cut and the bladder entered posteriorly. The anterior bladder mucosa is visualized and dissected to form the anterior lip of the bladder neck. Dorsal venous complex was controlled using diathermy. Circum-apical dissection of the urethra was performed and the urethra was then dissected a few mm distal to the prostatic notch after catheter withdrawal. The vesico-urethral anastomosis was performed with a running suture technique using two 23-cm 3/0 bared-synthetic absorbable monofilament sutures. The retroperitoneal space was packed with absorbable hemostats, tissue glue, and antiadhesives were applied. The posterior peritoneal incision was closed.
The patient was repositioned to the right lateral decubitus position, secured to the operating table and redraped before starting RAPN.
The surgical steps included bowel mobilization medially, liver mobilization with upper kidney pole exposure. After isolation of the main renal artery, the primary branches were dissected to identify the feeding tumor artery for selective clamping. The kidney was then freed from the perirenal fat to expose the renal tumor. Laparoscopic ultrasound was used intraoperatively by the bed-side assistant to identify tumor depth and margin. A selective lower pole arterial clamping was considered, 10-mg indocyanine green was injected followed by a 10-mL saline flush. Two short straight bulldog clamps were applied on the selected arterial branch. The Firefly fluorescence-imaging was used to test for adequacy of ischemia. Tumor excision was performed by cold cut scissors, and frozen section biopsy of renal parenchyma from the base of the operative bed, as well as the tumor edge, were obtained. Renorrhaphy was done using the sliding clip technique.
Total operative time was 240 minutes. The total console time for RS-RARP and RAPN was 61 and 71 minutes, respectively. RAPN warm ischemia time was 19 minutes. Estimated blood loss was 300 mL (RS-RARP, 50 mL and RAPN, 250 mL). There was no intraoperative complication and patient did not require any blood transfusion. Bowel function returned, and semisolid oral intake was resumed on postoperative day 1. The intraperitoneal drain, which was placed on the right paracolic gutter, was removed on postoperative day 2. The final pathology was prostatic adenocarcinoma GS 7 (3+4), and prostate weight was 30 gm, while for renal mass was clear RCC, Fuhrman grade 2 and pathologic tumor size was 1.6 cm×1.0 cm×0.9 cm. All surgical margins were negative. Length of hospital stay was 4 days.
Besides the well-known minimally invasive advantages of robotic-assisted surgery in the treatment of patients with urological malignancy compared to open and laparoscopic approaches, the combined procedure is advantageous to the patient precluding the need for readmission, and repeated induction of anesthesia, second procedure and associated higher cost. Moreover, it provides better cosmetic appearance by reducing the number of ports used compared to each procedure alone, and it minimizes risk of trocar injury.
Enhanced maneuverability of robotic platform has been one of its strength. This advantage has encouraged surgeons to venture into combined minimally invasive procedures. Jung et al. [6] pointed out in their report that though technically challenging robot-assisted laparoscopic single-site in the setting of simultaneous procedure, provided decreased overall hospital stay, avoidance of 2 separate procedures and minimized number of port placement. In the same manner, our recent study reusing ports from RS-RARP to perform RAPN was done.
Jung et al. [7] also reported the feasibility of combining bilateral partial nephrectomy and prostatectomy. They reported above-mentioned advantages and specifically warned readers towards the disadvantage of having prolonged anesthesia time and pneumoperitoneum. These disadvantages, however were avoided in our study having a short operative time, although direct comparison is not appropriate for previously reported case of Jung et al. [7].
Our recent approach using RS-RARP had a shorter operative time (240 minutes) in comparison to previous case reports (427 minutes) [8] and (442 minutes) [2] utilized the anterior conventional approach for radical prostatectomy. This shorter operative time decreases the risk of prolonged anesthesia exposure thus allowing us to perform combined approaches for synchronous tumor. In our previous report, we initially described our standard port placement technique for RS-RARP using 6 ports [5]. This port placement enabled us to use the same optical port for RS-RARP and RAPN which was different from what Patel et al. [2] reported. Moreover, application of RS-RARP technique added the benefits of shorter operative time and early continence recovery as previously reported [5].
To the best of our knowledge and vigorous literature search, there is no study done having direct comparison for combined procedure using Xi and Si system. We, therefore recommend that future comparison be done to portray advantages and disadvantages of both systems for combined cases. Further improvement of technique and enhancement of technology allow a well-experienced surgeon to achieve a better treatment option for patients with less morbidity.
Figures and Tables
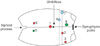 | Fig. 1Diagram illustrates ports placement during RS-RARP and right-side RAPN in the same setting. Red ports (were used for both procedures), blue ports (for RS-RARP) and green ports (for RAPN). 1, camera port (12 mm); 2–5, robotic port (8 mm); 6, assistant port (suction and liver traction, 5 mm); 7,8, assistant port (12 mm). RS-RARP, Retzius-sparing robot-assisted radical prostatectomy; RAPN, robot-assisted partial nephrectomy. Scan this QR code to see the accompanying video, or visit www.icurology.org or https://youtu.be/TJ3ZVPtRdCU. |
References
1. Ozsoy O, Fioretta G, Ares C, Miralbell R. Incidental detection of synchronous primary tumours during staging workup for prostate cancer. Swiss Med Wkly. 2010; 140:233–236.
2. Patel MN, Eun D, Menon M, Rogers CG. Combined robotic-assisted laparoscopic partial nephrectomy and radical prostatectomy. JSLS. 2009; 13:229–232.
3. Jung JH, Arkoncel FR, Lee JW, Oh CK, Yusoff NA, Kim KJ, et al. Initial clinical experience of simultaneous robot-assisted bilateral partial nephrectomy and radical prostatectomy. Yonsei Med J. 2012; 53:236–239.
4. Guttilla A, Crestani A, Zattoni F, Secco S, Dal Moro F, Valotto C, et al. Combined robotic-assisted retroperitoneoscopic partial nephrectomy and extraperitoneal prostatectomy. First case reported. Urologia. 2012; 79:62–64.
5. Lim SK, Kim KH, Shin TY, Han WK, Chung BH, Hong SJ, et al. Retzius-sparing robot-assisted laparoscopic radical prostatectomy: combining the best of retropubic and perineal approaches. BJU Int. 2014; 114:236–244.
6. Cagiannos I, Karakiewicz P, Eastham JA, Ohori M, Rabbani F, Gerigk C, et al. A preoperative nomogram identifying decreased risk of positive pelvic lymph nodes in patients with prostate cancer. J Urol. 2003; 170:1798–1803.
7. Jung JH, Kim HW, Oh CK, Song JM, Chung BH, Hong SJ, et al. Simultaneous robot-assisted laparoendoscopic single-site partial nephrectomy and standard radical prostatectomy. Yonsei Med J. 2014; 55:535–538.
8. John Hopkins Medicine. Partin tables [Internet]. Baltimore (MD): The Johns Hopkins University, The Johns Hopkins Hospital, and Johns Hopkins Health System;cited 2016 Feb 27. Available from: http://urology.jhu.edu/prostate/partintables.php.
SUPPLEMENTARY MATERIAL
An accompanying video can be found in the 'urology in motion' section of the journal homepage (www.icurology.org) or, be available on YouTube (https://youtu.be/TJ3ZVPtRdCU).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download