Abstract
We describe a case of a solitary functioning kidney with giant hydronephrosis secondary to ureteropelvic junction obstruction in a young girl who underwent successful robot-assisted tubularized flap pyelovesicostomy. The aim of this report was to highlight the feasibility and efficacy of this technique in salvaging such renal moieties and to present a brief review of the surgical options available for the management of giant hydronephrosis.
Giant hydronephrosis is an uncommon entity encountered in urological practice. Management options range from nephrectomy to various reconstructive strategies based on the anatomical configuration of the pelvicalyceal system and functional status of the kidney [1]. Since the turn of the 21st century, limited literature has emerged regarding the laparoscopic management of such complex conditions. The emergence of robotic assistance has been an immense help for such complex reconstructions. We describe a case of giant hydronephrosis in a solitary functioning kidney managed by robot-assisted tubularized flap pyelovesicostomy along with a brief review of the literature.
A 17-year-old girl presented with intermittent, dull, aching right flank pain for 2 months, along with low-grade fever for 5 days. Physical examination revealed a vague lump in the right lumbar region. Ultrasonography revealed gross hydronephrosis on the right side and a hypoplastic left kidney. Her serum creatinine was 1.4 mg/dL with a total leukocyte count of 10,700 cells/mm3. Following initial percutaneous nephrostomy (PCN) tube placement and resolution of her fever with antibiotics, a computed tomography (CT) nephrostogram with 3-dimensional reconstruction revealed giant hydronephrosis on the right side with ureteropelvic junction obstruction as evidenced by significant contrast retention after 30 minutes of study (Fig. 1). The renal pelvis reached up to the level of the 5th lumbar vertebra. Her bladder capacity was 300 mL.
She underwent robot-assisted Santosh-Post Graduate Institute tubularized flap pyelovesicostomy under general anesthesia by use of the da Vinci Si System (Intuitive Surgical, Sunnyvale, CA, USA) (Fig. 2). The patient was placed in the left lateral decubitus position. Following creation of pneumoperitoneum, the 12-mm camera port was inserted at the umbilicus. Two robotic ports and one assistant port were placed as shown in Fig. 2A. The colon was mobilized medially allowing exposure of the hydronephrotic kidney. The renal pelvis reached up to the sacroiliac joint. The peritoneum over the dome and anterolateral surface of the bladder was mobilized. A psoas hitch procedure was performed on the ipsilateral side by use of polyglactin 1/0 sutures. Cystotomy of around 3 cm in length was made on the anterolateral bladder wall followed by delivery of a supra-pubic catheter into the peritoneal cavity. A gap of 5 to 6 cm was noted between the renal pelvis and the bladder. An anterior trapezoid-shaped renal pelvic flap was created with its base at the most dependent portion of the pelvis. The flap dimensions were as follows: 7 cm length and widths of 5 cm and 3 cm at the base and apex, respectively. The posterior layer of the pyelovesicostomy anastomosis was closed by using Vicryl 3-0 sutures with a round body needle in an interrupted fashion with mucosato-mucosa approximation. The tip of the 16-Fr Foley catheter along with the bulb was placed inside the renal pelvis. The anterior layer of the anastomosis was similarly completed followed by tubularization of the flap over the catheter by use of full-thickness mucosa-to-mucosa sutures with Vicryl 3-0 on a round body needle in a continuous watertight fashion. An 18-Fr pelvic drain was placed. The operative time was 110 minutes.
The postoperative period was uneventful. The PCN was clamped on day 7. The drain was removed on day 5 and the patient was discharged with a strapped PCN and Foley catheter placed across the flap. The Foley splint was removed after 4 weeks. Follow-up CT nephrostogram after 6 weeks revealed patent anastomosis with prompt drainage of contrast into the bladder (Fig. 2H) and stable serum creatinine. At the 5-month follow-up, her serum creatinine was 1.3 mg/dL.
The earliest description of giant hydronephrosis dates to 1746 when Glass (quoted from [2]) recorded a hydronephrotic kidney containing 115 L of fluid. In 1939, Stirling defined giant hydronephrosis as a kidney containing more than 1 L of fluid [3]. Crooks et al. [4] gave radiographic criteria for the diagnosis, which included (1) kidney occupying the hemi-abdomen, (2) meeting or crossing the midline, and (3) about 5 vertebral bodies in length. Males are more commonly affected. Ureteropelvic junction obstruction is the most common cause (33%), followed by calculi (20%), congenital ureteral narrowing, ureteropelvic tumors, trauma, renal ectopia, retroperitoneal fibrosis, obstructive megaureter, ureteric atresia, and obstructive ectopic ureter with or without a duplex system [5]. Usually, the patient remains asymptomatic until the late stages because the disease progresses slowly [5] or may present with flank pain, fever, hematuria, or urinary tract infection. The huge hydronephrotic sac may be confused for hepatobiliary cysts, mesenteric cysts, cystic renal tumor, retroperitoneal tumors, ovarian cysts, or ascites [5]. Our patient luckily had consulted a physician at a younger age for her complaint of flank pain and fever; otherwise, her renal function might have further deteriorated.
The therapeutic approach is based on the initial presentation, the functional status of the affected and the contralateral kidney, and the anatomical configuration of the pelvicalyceal system [6]. Traditionally, a PCN tube is initially placed if the patient presents with fever, a nonvisualized or poorly delineated moiety on intravenous pyelography, or high serum creatinine. It further helps in assessment of 24-hour urinary creatinine clearance, prevents further renal damage, helps to stabilize renal function, and prevents postoperative acute renal injury secondary to sudden decompression [5]. In our case, the PCN tube was placed in view of suspected infected hydronephrosis, although the results of the culture were sterile. It additionally helped in performance of the CT nephrostogram, which provided precise anatomical details with avoidance of systemic exposure to contrast in the setting of a solitary functioning kidney. Preoperative decompression also allows safe placement of laparoscopic trocars without the fear of sac puncture and helps to provide ample space for instrument manipulation. It also allows time to manage postobstructive diuresis (in case of a solitary kidney or bilateral obstruction) preoperatively [1].
If the contralateral moiety is normal and previous surgical attempts have failed, nephrectomy may be considered. Nephrectomy should also be considered in cases of grossly hydronephrotic infected kidneys with poor function and a normal contralateral moiety [6]. However, nearly two-thirds of cases have preserved renal function and therefore are suitable for reconstruction. Reconstructive procedures should be considered in cases with significant residual renal function, a solitary functioning kidney, or bilateral disease. The high salvage rates are probably the result of a hugely dilated, compliant pelvicalyceal system. Because the drainage of urine in giant hydronephrosis is dependent on gravitational flow rather than peristalsis, the type of reconstruction procedure is partly based on the anatomical configuration of the pelvicalyceal system.
Various reconstructive procedures have been described for this clinical scenario [1678]. Options include reduction pyeloplasty in cases with a large extrarenal redundant pelvis, uretero-calycostomy or Boari-flap calycovesicostomy in cases of a grossly dilated inferior calyx with intrarenal pelvis, and pyelovesicostomy in cases of giant hydronephrosis with a dilated extrarenal pelvis reaching up to the bladder. Nephroplication and nephropexy are useful adjunctive procedures to reduce pelvicalyceal system stasis and to improve drainage, thereby reducing the risk of subsequent stone formation, infection, and deterioration in renal function [7].
One of the potential disadvantages of uretero-calycostomy is the formation of strictures and kinking of the anastomosis [8]. The Boari-flap calycovesicostomy ensures a wide anastomosis; however, vesicoureteric reflux with its deleterious impact on renal function is a major concern. In a study by Danforth et al. [9], however, no glomerular or tubular functional and histological abnormalities were noted in a canine model or in renal allotransplant patients with vesicopyelostomy or vesicocalycostomy.
Pyelovesicostomy has been previously described in cases of posttransplant donor ureteral necrosis, renal pelvic carcinoma, and ureteral carcinoma. It has also been described in cases of giant hydronephrosis as well as in a case of a solitary functioning pelvic kidney with ureteropelvic junction obstruction and recurrent renal calculi [1]. As described earlier, the presence of reflux following such reconstructions does not cause significant renal injury because the hugely dilated pelvicalyceal system bears the brunt of the reflux. Also, the reflux may not cause harm as long as the cultures are maintained sterile. Such refluxing reconstructive procedures can be chosen more freely in females than in males, who with increasing age may be more prone to develop infravesical obstruction owing to prostatic enlargement. The native ureter need not be disturbed, thereby limiting the extent of dissection needed. In an attempt to further dampen the impact of reflux on renal parenchyma in patients planned for pyelovesicostomy, we utilized the redundant renal pelvis to create a tubularized flap that was anastomosed to the urinary bladder instead of a direct anastomosis between the bladder and the most dependent part of the renal pelvis. This technique would also help in reducing the tension at the anastomotic site. Compared to a Boari-flap pyelovesicostomy or uretero-calycostomy, the creation of a renal pelvic flap is more straightforward without disturbing the native bladder tissue. The use of a Foley catheter as a splint ensured construction of a wide-caliber tubularized flap. It could easily be removed during follow-up without additional endoscopic intervention as would be needed for removal of a double-J stent.
With the emergence of minimal-access surgery, the morbidity associated with open surgery has been significantly reduced. Kumar et al. [1] described laparoscopic pyelovesicostomy as a salvage procedure for giant hydronephrosis secondary to ureteropelvic junction obstruction. Several other reports have described the feasibility and efficacy of complex reconstructive procedures performed laparoscopically. One of the major hurdles in laparoscopic reconstructive techniques is the difficulty in suturing during anastomosis. The robot-assisted surgery scores over the laparoscopic approach in this aspect. The ease with which intracorporeal suturing can be performed and the added advantage of 3-dimensional vision aided in successful performance of a complex reconstructive procedure with a good outcome.
Figures and Tables
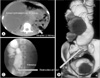 | Fig. 1(A) Axial section of noncontrast computed tomography (CT) scan showing grossly hydronephrotic right kidney with atrophic left kidney. (B, C) Nephrostogram with 3-dimensional CT reconstruction showing abrupt cutoff at L-5 vertebral level. Rt, right; Lt, left; PUJ, pelvi-ureteric junction. |
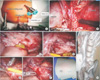 | Fig. 2Port placement (A), view following colon mobilization and dissection of renal pelvis (B), the renal pelvic flap with its base at the most dependent portion of the renal pelvis (C), posterior layer of tubularized flap pyelo-vesical anastomosis (D), closure of anterior layer over Foley catheter (E), completed anastomosis with tubularization of flap (F), postoperative wound (G), postoperative computed tomography nephrostogram after removal of splint showing prompt drainage of contrast in to the bladder (H). |
References
1. Kumar S, Pandya S, Acharya N, Agarwal M, Mandal AK, Singh SK. Laparoscopic management of atypical ureteropelvic junction obstruction: alternative and simple techniques for complex situations. Urol Int. 2008; 81:87–93.
2. Dennehy PJ. Giant hydronephrosis in a double kidney. Br J Urol. 1953; 25:247–251.
3. Chiang PH, Chen MT, Chou YH, Chiang CP, Huang CH, Chien CH. Giant hydronephrosis: report of 4 cases with review of the literature. J Formos Med Assoc. 1990; 89:811–817.
4. Crooks KK, Hendren WH, Pfister RC. Giant hydronephrosis in children. J Pediatr Surg. 1979; 14:844–850.
5. Sataa S, Kerim C, Sami BR, Nizar D, Rochdi E, Nidhameddine E, et al. Giant hydronephrosis in adult: what is the best approach? Retrospective analysis of 24 cases. Nephrourol Mon. 2011; 3:177–181.
6. Shah SA, Ranka P, Dodiya S, Jain R, Kadam G. Giant hydronephrosis: what is the ideal treatment? Indian J Urol. 2004; 20:118–122.
7. Hemal AK, Aron M, Wadhwa SN. Nephroplication and nephropexy as an adjunct to primary surgery in the management of giant hydronephrosis. Br J Urol. 1998; 81:673–677.
8. Mandal AK, Hemal AK, Vaidyanathan S. Boari flap calycovesicostomy: a salvage procedure for giant hydronephrosis due to ureteropelvic junction obstruction. J Postgrad Med. 1990; 36:38–40.
9. Danforth DN Jr, Javadpour N, Bergman SM, Terrill R. Pressure effects of urinary reflux studied with renal autotransplantation and pyelocystostomy. Urology. 1980; 15:17–22.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download