INTRODUCTION
Enterococci are a common cause of urinary tract infection and vancomycin-resistant
Enterococcus sp. (VRE) can cause a spectrum of disease including symptomatic infection, asymptomatic bacteriuria, and colonization of the urinary tract [
12]. Vancomycin resistance among enterococci is of increasing clinical significance. The economic impact of VRE infection and colonization is considerable and may be reduced by infection control measures [
3].
According to the European Antimicrobial Resistance Surveillance System, the prevalence of VRE in Europe varies from 1% to 30% and that in the United Kingdom (UK) varies from 20% to 30% [
4]. In the United States, the Centers for Disease Control and Prevention estimate approximately 30% of hospital enterococcal infections are VRE [
5]. Meta-analyses have concluded that vancomycin resistance is an independent predictor of mortality among hospital inpatients with enterococcal bloodstream infections [
6]. The adverse impacts of VRE bacteriuria are less clear [
7]. VRE colonization of hospital inpatients may be reduced by strict infection control measures [
8]. Reductions in the colonization rate may translate to reduced infection rates [
9].
Our surveillance program aimed to provide data regarding the prevalence of VRE in positive urine cultures, to identify risk factors for this, and to gather information regarding antibiotic susceptibility, which may be used to guide empirical treatment.
Go to :

MATERIALS AND METHODS
1. Ethics approval
This study was considered surveillance by the Health Research Authority. Therefore, formal ethical review or National Health Service Research and Development approval was waived.
2. Patient population
The computerized laboratory results database (MediTech) at Addenbrooke's Hospital in Cambridge, United Kingdom, was searched for urinary isolates of all pathogens including Enterococcus sp. for the period 1 January 2005 to 31 October 2014. Urine samples received from outside our institution (including community isolates) were excluded from the analysis. Of the total number of samples received each year, approximately 1% contained incomplete records, 1%–2% contained unsuitable specimens, 5%–7% were from patients aged less than 16 years (children), and 1%–2% were of heavy mixed growth and indicated likely contamination; all of these were excluded.
In this time period, we identified and reviewed 5,528 urine cultures from 5,090 different patients. Patient information such as age, sex, specimen type, and whether the patient was an inpatient at the time of collection was collected. We excluded patients aged <16 years because pediatric populations have treatment guidelines that differ from those for adult populations. Samples ordered less than 30 days after a previous sample were not counted because they would skew our data by analyzing the same infection multiple times. Unusual specimen types including, for example, nephrostomy, ileal conduit, extraprostatic secretion, suprapubic aspirate, or bag specimens were not included because they each represent distinct clinical scenarios.
3. Culture analysis
Urine was processed by calibrated loop sampling onto chromogenic clear media (Oxoid Ltd., Basingstoke, UK). A positive culture was defined as ≥105 colony-forming units (CFU)/mL except for samples from children and pregnant women for which a cutoff value of >103 CFU/mL was used. Susceptibility testing was performed by British Society of Antimicrobial Chemotherapy disc diffusion testing and reported for ampicillin, nitrofurantoin, linezolid, quinupristin/dalfopristin, and tigecycline. Susceptibilities that were classified as "intermediate resistance" were redesignated as "sensitive." Enterococcus faecium and Enterococcus faecalis were only distinguished from 2012 onward and were previously reported as Enterococcus sp.
4. Statistical analysis
To assess risk factors (age, sex, catheter
in situ, and inpatient status) on the probability of having VRE, we fitted a logistic regression model with the variables entered simultaneously. Non-VRE was the comparator. Pairwise interactions were assessed by inclusion in the model; however, none were found to be significant and none were included in the final model. VRE probability was found to vary nonlinearly with age; hence, age was entered as a restricted cubic spline with knots at the tertiles. Cuzick test for trend was used to examine trends in VRE-positive cases across the years [
10]. Comparisons of antibiotic susceptibility between
Enterococcus subtypes were made by using Fisher exact test. Data management and analysis was performed by using Stata SE 12.0 (StataCorp LP., College Station, TX). All tests were two-sided with significance set at p<0.05.
Go to :

RESULTS
In total, 5,528 cultures (14.7% of all specimens in our database) were positive for
Enterococcus sp., of which 542 (9.8%) were vancomycin-resistant. The total number of enterococcal urine cultures ranged from 451 in 2013 to 707 in 2007. The proportion of vancomycin resistance among enterococcal urine cultures varied from a high of 13.9% in 2006 to a low of 7.1% in 2013 but did not change significantly over the period (p>0.35) (
Fig. 1).
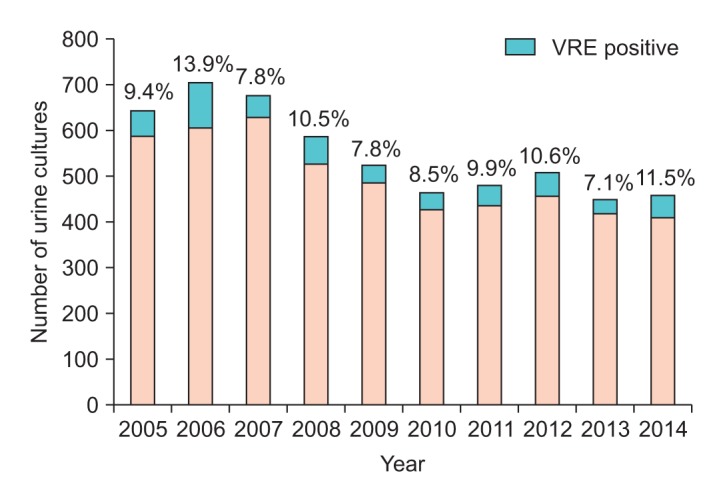 | Fig. 1Number of urine cultures positive for Enterococcus sp. each year. The proportion that demonstrated vancomycin resistance is shaded in red and the percentage is shown above the bars. The proportion that was vancomycin susceptible is shaded in blue. No statistically significant trend in VRE percentage over the years was observed (p>0.35). VRE, vancomycin-resistant Enterococcus sp.
|
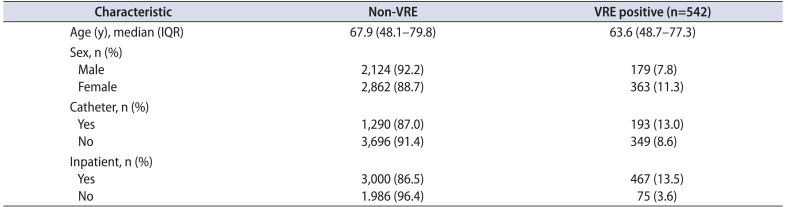
Compared to patients with non-VRE, VRE patients' median age was 4 years younger (63.6 years vs. 67.9 years). Female patients, those catheterized, and inpatients were more likely to have VRE (
Table 1). After adjustment for age and the other categorical variables, female sex and inpatient status were independent, significant predictors of VRE urinary infection (p<0.001). Females were observed to have a 50% higher odds of VRE than males and inpatients were at 4 times the risk of VRE than were those who were tested as outpatients (
Table 2).
Table 1
Characteristics of patients with VRE compared to urine cultures positive for non-VRE
|
Characteristic |
Non-VRE |
VRE positive (n=542) |
|
Age (y), median (IQR) |
67.9 (48.1–79.8) |
63.6 (48.7–77.3) |
|
Sex, n (%) |
|
|
|
Male |
2,124 (92.2) |
179 (7.8) |
|
Female |
2,862 (88.7) |
363 (11.3) |
|
Catheter, n (%) |
|
|
|
Yes |
1,290 (87.0) |
193 (13.0) |
|
No |
3,696 (91.4) |
349 (8.6) |
|
Inpatient, n (%) |
|
|
|
Yes |
3,000 (86.5) |
467 (13.5) |
|
No |
1.986 (96.4) |
75 (3.6) |

Table 2
Logistic regression to predict VRE vs. non-VRE with all variables entered simultaneously with age as a restricted cubic spline

|
Variable |
Odds ratio (95% CI) |
p-value |
|
Sex, female vs. male |
1.55 (1.28–1.89) |
<0.001 |
|
Catheter, yes vs. no |
1.15 (0.94–1.40) |
0.16 |
|
Inpatient, yes vs. no |
4.16 (3.21–5.39) |
<0.001 |

Our data showed emerging resistance to nitrofurantoin with rates climbing from near zero to above 40% (
Fig. 2). Ampicillin resistance varied somewhat but increased from 52% in 2006 to over 80% from 2009 onward. No trends were observed for tigecycline or linezolid, which both remained highly effective against VRE across the study period.
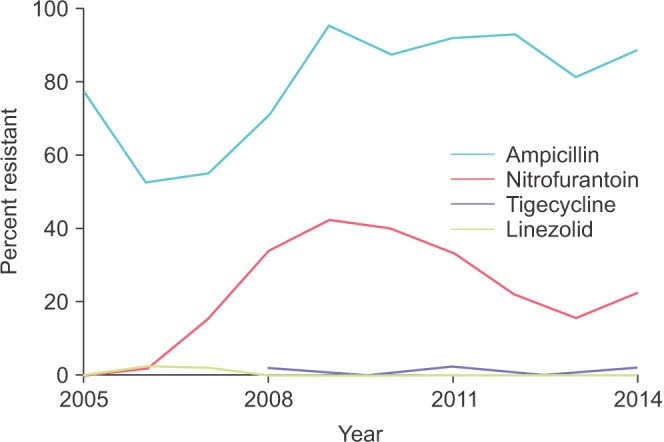 | Fig. 2Antibiotic resistance of VRE urinary isolates across time. The blue, red, orange, and green lines represent ampicillin, nitrofurantoin, tigecycline, and linezolid, respectively. VRE, vancomycin-resistant Enterococcus sp.
|
From 2012, reliable species identification of
Enterococcus sp. was available. A total of 15 of 928
E. faecalis specimens (1.6%) were vancomycin-resistant, whereas 107 of 209
E. faecium species (51.2%) were vancomycin-resistant.
E. faecium but not
E. faecalis was almost entirely resistant to ampicillin (p<0.01) (
Fig. 3). Nitrofurantoin was more effective against
E. faecalis compared to
E. faecium (p<0.05). No significant difference was observed between
E. faecium and
E. faecalis for linezolid and tigecycline; susceptibility was near 100% for both subtypes to these antibiotics.
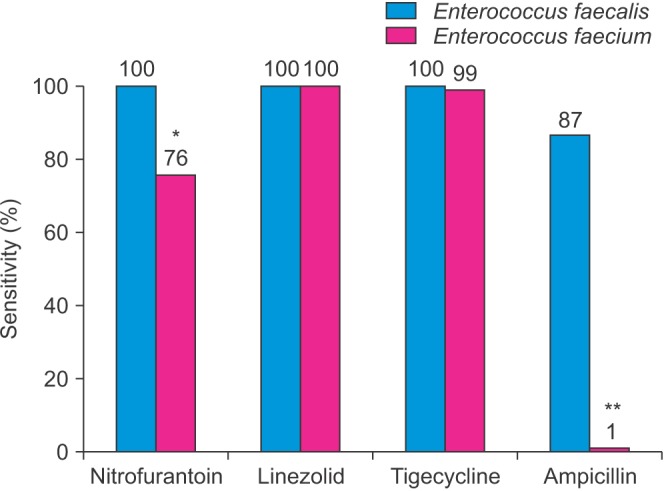 | Fig. 3Antibiotic susceptibility profile of vancomycin-resistant Enterococcus faecalis (n=15), shaded in blue, and E. faecium (n=107), shaded in red, from 2012 onward. *p<0.05. **p<0.01.
|
Go to :

DISCUSSION
To date, there have been relatively few large-scale studies considering VRE antibiograms specific to urinary tract infection and we believe our study represents the second largest. The largest study was conducted in 2002 across 38 medical centers in North America with 27,145 isolates of
Enterococcus sp., 697 of which were VRE [
11]. The principal advantage of our study is that our data were collected over a decade and allowed us to observe any changes during that time.
We have reported that the incidence of vancomycin resistance among enterococcal urinary isolates varied from 8% to 14% per year without a statistically significant trend across time (
Fig. 1). A multicenter study based in the United States in 2002 reported incidences varying from 2% to 20% between different institutions [
11]. The steady incidence we observed may be somewhat reassuring to clinicians concerned by antibiotic resistance becoming more widespread.
In 2005 nitrofurantoin susceptibility was near 100%, which closely agrees with Zhanel et al. [
11]; it then dropped to below 60% and then increased to 80% (
Fig. 3). For ampicillin, resistance also gradually increased, although it fluctuated from 52% to 95%, which broadly concurs with Zhanel et al. [
11]'s recorded resistance of 86%. In addition, we reported <1% resistance for linezolid, which is concordant with this previous study. Tigecycline is a relatively new broad-spectrum antimicrobial agent and has demonstrated efficacy in treating VRE bacteremia but not bacteriuria [
12]. Although we observed near 100% susceptibility, our data do not necessarily translate into clinical efficacy because the urine pharmacokinetics of tigecycline are controversial [
13].
The variations we observed illustrate the significant variations antibiotic resistance can undergo across time. Additionally, although useful for uncomplicated VRE urinary tract infection, nitrofurantoin is perhaps not as reliable as it was in 2005. This also serves to remind clinicians that the landscape of antibiotic resistance is unpredictable and that surveillance programs are essential to monitor for any unexpected deviations.
We identified female sex, urinary catheterization, inpatient status, and younger age as risk factors for VRE-positive urine culture (
Tables 1,
2). We were unable to identify any publications regarding risk factors specifically for VRE bacteriuria, but it has been established that risk factors for other VRE infections include intravascular or bladder catheter devices, medical comorbidities, prolonged hospitalization, and residential care [
14]. Unfortunately, this information was not captured in our database. Unexpectedly, the risk factors we identified differed from those identified for other uropathogens, such as fungal urinary tract infection or extended-spectrum beta-lactamase (ESBL)-producing
Enterobacteriaceae [
1516]. This difference may result in differing mechanisms in how these resistant organisms are spread, as VRE is often spread between inpatients and may be reduced by infection-control measures such as handwashing [
17], whereas ESBL is typically acquired after antibiotic usage or travel to endemic areas [
18].
Vancomycin-resistant
E. faecium were very resistant to ampicillin, whereas vancomycin-resistant
E. faecalis were very susceptible (p<0.01) (
Fig. 3), which is concordant with the findings of Zhanel et al. [
11]. In addition, we observed that nitrofurantoin was more effective against
E. faecalis (p<0.05), whereas the study by Zhanel et al. [
11] found no difference.
The retrospective nature of this study limited our ability to correlate the clinical data with the pathology results. Our study included all positive urine cultures and it was not possible to distinguish between different infections or asymptomatic bacteriuria. It is thought that the susceptibility profile of some uropathogens varies depending on the clinical severity of the infection. However, owing to the large size of our cohort we feel it is reasonable to assume the proportions of infection and asymptomatic bacteriuria remained fairly constant and were not responsible for differences between years. Our results are only from a single center and may not accurately reflect the situation at other hospitals in our region. Earlier studies have shown that susceptibility profiles vary significantly between similar institutions [
11]. None of these limitations diminish our ability to demonstrate increases in antibiotic resistance as we have shown this longitudinally across time. Our data were also limited by the types of antibiotics tested by the laboratory, because no tests were undertaken for fosfomycin, which would have been of interest.
Several review articles have suggested treatment protocols for VRE urinary tract infection. For uncomplicated VRE cystitis, Heintz et al. [
2] recommended ampicillin or amoxicillin as the first-line treatment. Our results indicate that susceptibility to ampicillin has dropped over time to currently less than 20% of all enterococcal isolates. However, ampicillin may be reserved for known cases of
E. faecalis only. Nitrofurantoin may be a more reliable initial option, which agrees with recommendations by other authors and is consistent with the Australian therapeutic guidelines for acute cystitis in adults [
141920]. The high level of susceptibility to linezolid we have demonstrated supports recommendations that this antibiotic may be relied upon to treat VRE urinary tract infection in complicated cases such as pyelonephritis, urinary tract infection with bacteremia, or urosepsis [
1211].
Despite our focus on antimicrobial therapy, it must be remembered that source control, such as abscess drainage or catheter removal, is an integral component of treatment [
21]. Additionally, a positive VRE urine culture does not itself necessitate antibiotic treatment as this may indicate a contaminated specimen or asymptomatic bacteriuria [
12].
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download